Katya Zlatkova a*,
aAlumna, Applied Psychophysiology, Saybrook University, Pasadena, California, United States;
*Katya Zlatkova, kzlatkova@saybrook.edu
For citation: Zlatkova, K.M. (2025) Neurofeedback for brain dysregulation in autism: QEEG and swLORETA case findings in the context of frequent screen exposure.Nootism 1(4), 55-68, https://doi.org/10.64441/
Abstract
Excessive screen exposure during early development has been linked to significant neurophysiological and behavioral changes, particularly in children with autism spectrum disorder (ASD). This paper reviews current literature on the neurophysiological mechanisms underlying screen overuse and screen addiction, highlighting alterations in brain regions involved in reward processing, executive function, emotional regulation, and habit formation. Drawing on electrophysiological, neuroimaging, and behavioral evidence, it outlines how prolonged screen use can result in maladaptive neuroplasticity, diminished reward sensitivity, and cognitive-emotional dysregulation.
To illustrate these effects in a real-world context, a single-subject case study is presented. The subject, a 7-year-old male with autism spectrum disorder (ASD) and a history of frequent screen exposure, underwent a full-cap, 19-channel quantitative electroencephalography (QEEG). Functional connectivity and source analyses were conducted using NeuroGuide’s NeuroNavigator (Applied Neuroscience, Inc.), which applies standardized weighted low-resolution electromagnetic tomography (swLORETA) algorithm. Pre-intervention findings revealed elevated slow-wave activity in the frontal regions, excessive high-frequency activity in the posterior areas, and dysregulation across multiple neural networks, including the default mode network (DMN), reward system, executive circuits, and amygdala. Following 68 sessions of individualized surface neurofeedback training based on the QEEG findings, post-intervention measures showed normalization in spectral patterns and improved functional connectivity. These neurophysiological changes corresponded with clinically significant behavioral improvements, including increased verbal output, reduced emotional outbursts, greater task independence, and more regulated screen use. Findings from this case suggest that neurofeedback can be an effective intervention for addressing screen-related cognitive and emotional regulation challenges in children with ASD.
Keywords: screen addiction, neurofeedback, QEEG, swLORETA, functional connectivity, autism spectrum disorder (ASD), neurodevelopment, reward system, emotional regulation
Introduction
Modern existence is unimaginable without the use of technology and screen devices. While these tools offer benefits and convenience, excessive screen use can become problematic and lead to addiction. Screen overuse refers to spending too much time on screens than recommended by health guidelines, without compulsive symptoms. In contrast, screen addiction (also called digital addiction) is a non-formal term used to describe behavioral patterns associated with obsessive use of screen-based devices such as tablets, smartphones, computers and televisions. Although not formally classified in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), some related conditions such as gaming disorder, internet addiction disorder and problematic smartphone use are recognized due to their associations with distress, impairment and negative outcomes.
While the terms screen overuse and screen addiction are used interchangeably, they have a distinct meaning on the behavioral spectrum. What distinguishes screen overuse from screen addiction are the symptoms of dependency. These include constant preoccupation with screen use, lack of self-control, high tolerance for time spent on the screen, neglect of other daily activities, and withdrawal symptoms (i.e. anxiety, irritability, and anger). Such symptoms can disrupt emotional well-being and psychological health, especially in young children and adolescents, whose brains are still wiring and have not reached developmental maturity. Recent studies have proposed that prolonged screen exposure may contribute to neurodevelopmental alterations in vulnerable populations, particularly in children with autism spectrum (Vezenkov & Manolova, 2024; 2025a; 2025b; Manolova & Vezenkov, 2025).
Leading health organizations have established guidelines to protect neurodevelopmental health. For example, children under 18 months are advised to avoid screen exposure entirely, while children aged 2–5 should limit screen time to one hour per day of quality content. For children ages 6 and older, screen use should be limited and managed with consistent boundaries that prioritize sleep, physical activity, and face-to-face interactions (American Academy of Pediatrics, 2016; Mayo Clinic, 2023; Centers for Disease Control and Prevention, 2023). Despite these recommendations, many young children far exceed these limits; alarming reports show that infants today average two to three hours of screen time daily (Chen & Adler, 2019), and nearly 90% of toddlers are regularly exposed to digital media (Huang et al., 2024).
Current research increasingly demonstrates evidence that screen overuse and screen addiction directly impact brain function. The neurophysiology of screen addiction involves alterations within the circuitry of interconnected brain areas and neurochemical changes. These include changes within the dopaminergic reward system, the prefrontal cortex (PFC), the default mode network (DMN), anterior cingulate cortex (ACC), the amygdala and processes underlying neuroplasticity (See Table 1). The purpose of this paper is to discuss the neurophysiological basis of screen addiction and examine how it contributes to behavioral and emotional dysregulation in youth. To illustrate these effects in a real-world context, this paper presents a case study of a child diagnosed with ASD and a history of extended screen use. Neurofeedback was used as an intervention to support neuroregulation and improve functional brain connectivity. Neurofeedback is a therapeutic technique that teaches individuals how to modulate their brainwave activity by providing real-time feedback of their neural oscillations.
The Reward System
During electronics use, social media and gaming, the brain releases a rush of dopamine, aka “the feel-good” neurotransmitter. This dopamine release activates the brain’s mesolimbic reward system, a key factor in addiction development. While engaging with digital technologies, dopamine neurons in the ventral tegmental area project via efferent nerves to the nucleus accumbens (NAcc) which when excited signals the prefrontal cortex (PFC) to increase dopamine production (Chen et al., 2023). This activates the reward system which reinforces the behavior to achieve sensations of pleasure. Over time, repeated stimulation leads to abnormal activity within the NAcc. However, prolonged excessive screen time overstimulates these reward pathways and can reduce dopamine receptor availability. This “blunted” reward response means people need more stimulation to experience satisfaction (Volkow et al., 2016).
Koepp et al. (1998) provided direct evidence that video games can trigger dopamine release in the brain. The researchers measured the levels of dopamine in the ventral striatum via positron emission tomography (PET) with a dopamine binding radioligand while their participants played video games. The results from this experiment showed that the magnitude of dopamine release was comparable to levels seen in response to stimulant drugs. Ultimately, screen-based stimuli activate the mesolimbic dopamine pathway, producing reward and pleasure in ways that mimic traditional addictions. Over time, repeated exposure can desensitize dopamine pathways contributing to cravings, increasing tolerance, and reinforcing compulsive use.
The Prefrontal Cortex
Neuroadaptation in the reward circuits due to screen addiction comes along with functional changes in the prefrontal cortex (PFC), a region in the brain responsible for executive functioning, decision making, impulse control and inhibition. The PFC is closely linked to dopaminergic input; thus, overstimulation and excessive digital use can impair its normal functioning. This results in behaviors such as heightened emotional reactivity, poor self-control and lack of motivation for non-screen activities. Hutton et al. (2020) assessed white matter integrity in healthy children ages 3-5 (N=47). Using diffusion tensor imaging (DTI), the researchers examined the participants’ white matter tracts while their parents completed the ScreenQ questionnaire, which measured and quantified screen use. Higher ScreenQ scores (higher screen usage) were associated with a) lower white matter microstructural integrity, especially in areas supporting language and literacy skills, such as the prefrontal cortex and b) reduced organization and myelination of white matter fibers, which are found crucial for efficient cognitive processing. These findings suggest that increased screen time in preschool-aged children may be linked to alterations in brain structure, potentially impacting language development and executive functions. Furthermore, they emphasize the importance of moderated screen use in early childhood, aligning with recommendations by health organizations such as the American Academy of Pediatrics. These results encourage setting screen time limits to promote interactive, non-screen-based activities and to support healthy brain development. Nevertheless, excessive screen use is associated with reduced PFC activation, impairing functions such as self-regulation, attention and long-term planning. This condition mirrors patterns seen in substance addiction and attention deficit hyperactivity disorder, suggesting overlapping neural mechanisms.
Default Mode Network
During brain activation, groups of neural regions and nodes which form networks get activated. The default mode network (DMN) includes the following key areas: the medial prefrontal cortex, posterior cingulate cortex and precuneus. This network is activated during rest, when the individual is not engaged with the environment, during daydreaming, mind-wandering, recalling memories, thinking about the future, or reflecting on oneself and others. A well-functioning DMN supports healthy processing of emotions and helps regulate mood. Dysregulated internal focus and reward-seeking behaviors such as screen addiction can lead to functional connectivity alterations within the DMN (Lou et al., 2019).
Lou et al. (2019) studied 24 college students with identified mobile phone addiction (MPA) and 16 age- and gender-matched healthy controls. Resting-state functional magnetic resonance imaging (fMRI) was used to assess functional connectivity (FC) within the DMN, focusing on the posterior cingulate cortex (PCC) as a central node. Participants completed several psychological scales, including the Self-Rating Anxiety Scale (SAS), Self-Rating Depression Scale (SDS), Barratt Impulsiveness Scale-11 (BIS-11), and Rosenberg Self-Esteem Scale, to evaluate emotional and personality traits.
The results from this study demonstrated that students with MPA exhibited significant alterations in resting-state FC within the DMN, particularly between the PCC and other regions such as the medial prefrontal cortex (mPFC), inferior parietal lobule, and hippocampus. The degree of altered FC in the DMN correlated positively with higher scores on anxiety, depression, and impulsivity scales, and negatively with the self-esteem score. These findings suggest that excessive mobile phone use may disrupt the normal functioning of the DMN, potentially leading to emotional dysregulation, impaired self-referential processing, reduced focus and increased rumination.
Anterior Cingulate Cortex
The anterior cingulate cortex (ACC) is another key area affected by screen overuse. The ACC is implicated in attention regulation, error detection, emotional control, and conflict monitoring. In their systematic review, Méndez et al. (2024) synthesized findings from functional magnetic resonance imaging (fMRI) studies and examined how internet and smartphone addiction impact cognitive control in adolescents and young adults. Individuals with internet or smartphone addiction consistently exhibited abnormal activation patterns throughout the studied fMRI datasets. Specifically, alterations were observed in the ACC, insula, amygdala, dorsolateral prefrontal cortex (DLPFC), and frontal and parietal lobes. These findings correlate with impaired executive functions like inhibitory control and error monitoring. The neural alterations observed in addicted individuals resemble those seen in substance use disorders, supporting the concept of behavioral addictions having similar neurobiological bases. These brain changes may explain the difficulties addicted adolescents and young adults have in controlling their screen use, maintaining attention, and regulating emotions. Taken together, these findings highlight the importance of early interventions that support cognitive and emotional regulation in young populations at risk of screen-related addictions.
Amygdala
Research suggests that increased screen exposure in early childhood may disrupt the functional organization of brain networks associated with cognitive control and emotional regulation. One structure central to these functions is the amygdala. The amygdala, involved in emotional salience and fear responses, may become hypersensitized in screen addiction leading to elevated stress and anxiety when access to screens is restricted (withdrawal symptoms).
A longitudinal study by Weissman et al. (2021) investigated how screen time during early childhood (ages 12–36 months) affects brain network development and socio-emotional outcomes by age 7. The researchers found out that higher screen use in toddlers was associated with greater integration between the cognitive control and emotion-processing networks (including the amygdala and prefrontal cortex) by age 6. This hyperconnectivity is atypical for healthy brain maturation; it was linked to difficulty regulating emotions and forming social bonds by age 7, including lower empathy and challenges with peer interaction. Early screen exposure also altered the typical development of brain systems responsible for emotional regulation and executive function. However, children who had higher screen time but also engaged in more frequent reading with caregivers showed less disruption in brain network development and better socio-emotional outcomes. Interactive reading with caregivers acted as a protective environmental factor, possibly by supporting language, social, and emotional skills, and promoting healthier brain development. Therefore, shared parent–child reading time during early childhood significantly moderates the effects of screen time, emphasizing the importance of interactive, non-screen-based activities in promoting healthy neurodevelopment.
Neuroplasticity and Habits Formation
Chronic screen use and screen addiction can rewire neural circuits through maladaptive neuroplasticity, strengthening automatic behaviors such as cue-response patterns (such as checking phones upon notification), reducing sensitivity to offline rewards (i.e., in-person interaction), and interfering with habit formation and healthy neuroplasticity. Functional neuroimaging and electrophysiological evidence suggest that individuals with internet gaming disorder (IGD) exhibit diminished neural responsiveness to real-life social rewards (Nie et al., 2025). They underscore the impact of chronic screen use on the brain's reward and habit-forming circuits, highlighting the importance of monitoring and moderating screen time to reduce potential neurobiological consequences.
In a recent event-related potential (ERP) study, Nie et al. (2025) found that individuals with IGD demonstrated significantly blunted contingent negative variation (CNV) amplitudes during the anticipation of socially rewarding stimuli (e.g., smiling faces), relative to healthy controls. Blunted CNV responses indicate lower motivation toward social cues, which may help explain the withdrawal from real-life interaction seen in IGD. This neurophysiological pattern reinforces a preference for digital environments. Moreover, the degree of blunting correlated with symptom severity, indicating that this neural pattern may serve as a biomarker of disorder intensity.
Chen et al. (2023) reviewed neurophysiological data from 8,324 participants (ages 9-11) in the Adolescent Brain Cognitive Development (ABCD) study and examined their fronto-striatal circuitry, a brain network essential for self-regulation and impulse control. The results revealed that:
- a) Children with higher daily screen time exhibited weaker connectivity between the frontoparietal network and the striatum, indicating diminished development of inhibitory control mechanisms; and
- b) They also showed a stronger preference for immediate rewards over delayed gratification, suggesting heightened reward sensitivity.
In addition, altered connectivity in the dorsal striatum, associated with habit formation, suggested that excessive screen use might lead to habitual seeking behaviors. Taken together, the data suggests potential long-term negative effects of excessive screen time on children's neurodevelopment, especially in brain regions governing self-control and habit formation.
Table 1
Summary of Different Brain Areas Affected by Screen Addiction or Screen Overuse
| Brain region | Function | Impact of Excessive screen use | Supporting studies |
| Default Mode Network (DMN) (mPFC, PCC) | Resting state, self-referential thought | Altered connectivity, emotional dysregulation | Lou et al. (2019) |
| Anterior Cingulate Cortex (ACC) | Attention, error monitoring, emotional control | Impaired cognitive control and inhibition | Méndez et al. (2024) |
| Amygdala | Emotional salience, fear responses | Hyperconnectivity, anxiety, withdrawal symptoms | Weissman et al. (2021) |
| Mesolimbic Dopamine Pathway (NAcc, VTA) | Reward, pleasure, motivation | Dopamine dysregulation, tolerance, craving | Koepp et al. (1998), Volkow et al. (2016) |
| Prefrontal Cortex (PFC) | Executive functions, impulse control | Reduced activation, poor self-regulation | Hutton et al. (2020) |
| Fronto-striatal Network | Self-regulation, habit formation | Weakened inhibitory control, habitual behavior | Chen et al. (2023) |
Neuroprotective Factors and Mitigation Strategies
Although the neurophysiological risks of excessive screen use are well documented, emerging research points to several neuroprotective factors that can help buffer these effects. For example, outdoor play has been linked to improved sensorimotor integration, emotional regulation, and attention span (Gray, 2011). Exposure to natural light also supports circadian rhythm development and healthy sleep patterns (Cheung et al., 2017). Mindfulness-based interventions in children and adolescents have been associated with increased prefrontal activation, better emotional regulation, and reduced impulsivity (Zenner et al., 2014).
Parental mediation, particularly active involvement including co-viewing and guided conversations, has been shown to reduce the risks of problematic media use and enhance children's cognitive and emotional development (Nathanson, 2001; Nikken & Jansz, 2006). Moreover, shared interactive experiences, such as reading aloud, board games, and arts-based activities, foster social bonding, language acquisition, and cognitive flexibility (Weisleder & Fernald, 2013).
In addition to these behavioral and environmental strategies, biofeedback and neurofeedback are promising interventions for restoring self-regulation and functional connectivity in children affected by excessive screen use. For example, by providing real-time feedback on brainwave activity, neurofeedback supports the modulation of dysregulated neural patterns and strengthens cortical networks involved in attention, emotional regulation, and executive function (Arns et al., 2009; Coben & Myers, 2010; Vezenkov & Manolova, 2024; 2025a; 2025b). These protective practices counteract the adverse effects of screen exposure. They also strengthen neural pathways essential for executive function, self-regulation, and social-emotional growth.
To illustrate how neurofeedback can support neurophysiological recovery, the following study is presented.
Case Study
This case involves a 7-year-old male, referred to here as Leo G. (pseudonym), who was diagnosed with autism spectrum disorder (ASD) in early childhood and had a history of frequent screen exposure. Although Leo received a formal diagnosis in 2020, developmental red flags began emerging as early as 12 months of age, with delayed speech development delayed speech development as the most prominent concern. Leo was evaluated and treated in a private clinical practice in Illinois, USA. Leo’s neurophysiological and clinical presentation demonstrated patterns described in recent literature on the impact of prolonged screen use in developmentally vulnerable children, including the biomarker profiles proposed by Vezenkov and Manolova (2025a). Their work identifies a characteristic set of QEEG and functional connectivity features, including excessive slow-wave activity in frontal regions, elevated high-frequency activity in posterior areas, and dysregulation within the default mode, reward, and emotional processing networks that may reflect addiction-like neuroplastic changes associated with early and excessive screen exposure.
Leo’s family provided pre- and post-training self-reports to support clinical evaluation. At the time of the assessment his parents reported he experienced persistent under-arousal, apathy, low motivation, emotional dysregulation, and limited speech production. They also noted that Leo consistently required assistance with tasks they believed he was capable of completing independently. Leo also appeared to rely on screens as a means of self-soothing and emotional regulation. His parents reported that screen use was one of the few strategies that consistently helped him calm down, particularly during moments of frustration, sensory overload, or transitions.
Leo’s first full cap 19-channel quantitative EEG (QEEG) was conducted in June 2024 using Deymed amplifier. The recording lasted 15 minutes in the eyes-open condition; eyes-closed data could not be obtained. During the assessment, Leo watched videos on his mother’s smartphone, which helped him remain calm and cooperative throughout the session.
The EEG recording was analyzed and quantified using NeuroGuide (Applied Neuroscience, Inc.) and compared to a normative database of healthy individuals of Leo’s age. Functional connectivity and source analyses were conducted using NeuroGuide’s NeuroNavigator module which applied the standardized weighted low-resolution electromagnetic tomography (swLORETA) algorithm to estimate cortical current density and identify dysregulated brain regions.
Leo’s QEEG and functional connectivity findings closely mirrored the biomarker patterns described by Vezenkov and Manolova (2025a) in children with prolonged screen exposure, including disruptions in functional connectivity, cortical over- or under-arousal, and profiles associated with addiction-like neuroplastic patterns.
Several notable findings were observed. Prominent slow-wave activity was seen in the raw EEG (see Figure 1). The QEEG assessment revealed elevated slow-wave activity (1–5 Hz) in the prefrontal, frontal, and temporal regions Additionally, increased high-frequency activity (25–29 Hz) was noted in the parietal and occipital areas, as well as hypercoherence in the delta frequency band (see Figure 2, left image). These electrophysiological patterns are consistent with cortical under-arousal in anterior regions and overactivation in posterior areas, aligning with profiles associated with prolonged screen exposure.
Figure 1. Slow-Wave Oscillations in the Raw EEG
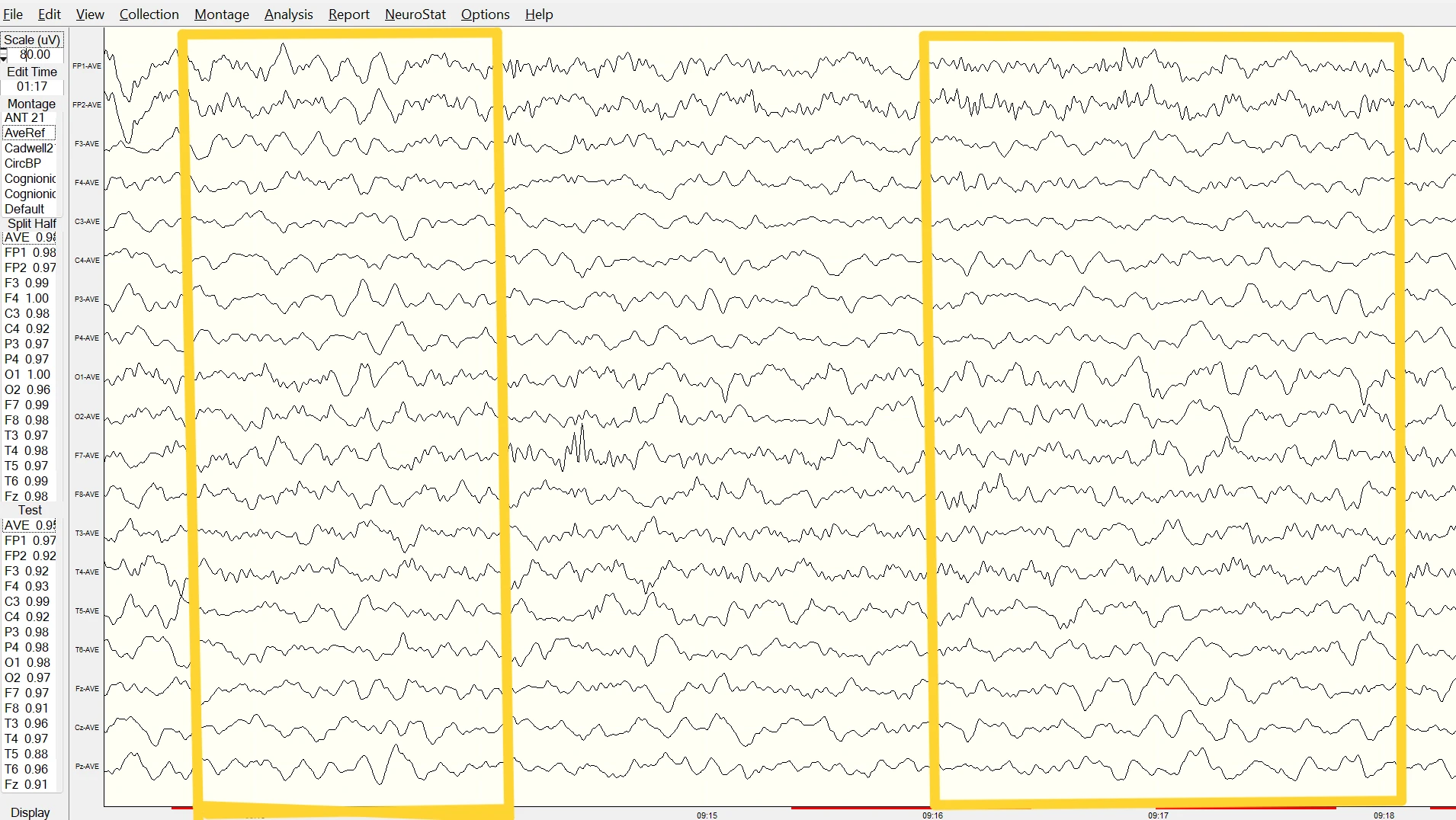
Note. Raw EEG trace showing slow-wave activity (around 4 Hz) during eyes-open baseline, recorded using a Deymed amplifier. Data reviewed in NeuroGuide.
Figure 2. Pre (Left Image) and Post (Right Image) Quantitative EEG
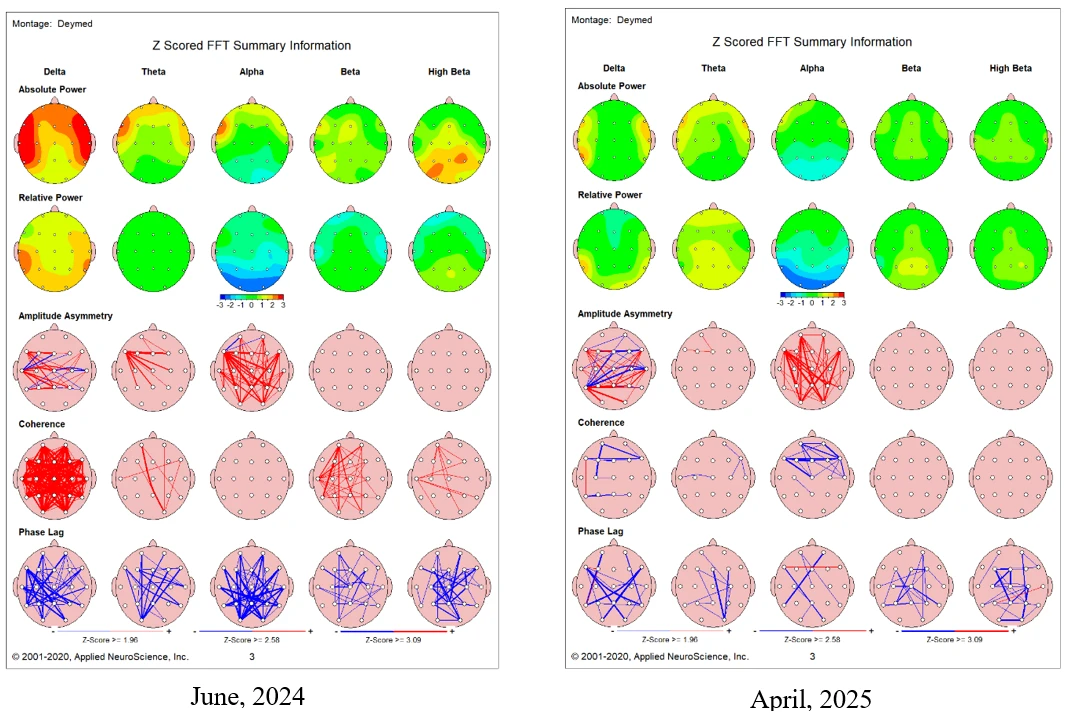
Note. QEEG images illustrate changes in brainwave activity before and after 68 sessions of neurofeedback. Data was analyzed using NeuroGuide software (Applied Neuroscience, Inc.).
Functional Connectivity
In Leo’s pre-training functional connectivity, z-score deviations ≥ 2.0 were observed in multiple networks, including the default mode, reward, and executive function circuits. These findings were used to identify training targets and monitor neurophysiological change across the intervention. Post-training reductions in z-scores toward the normative range reflect a trend toward improved functional regulation.
One specific finding was atypical connectivity within the default mode network (DMN), particularly in Brodmann areas (BA) 11L, 11R, 30L, 35L, and 35R (see Figure 3).
Figure 3. Functional Connectivity Within the Default Mode Network: Most Abnormal Deviations Observed at 2 Hz
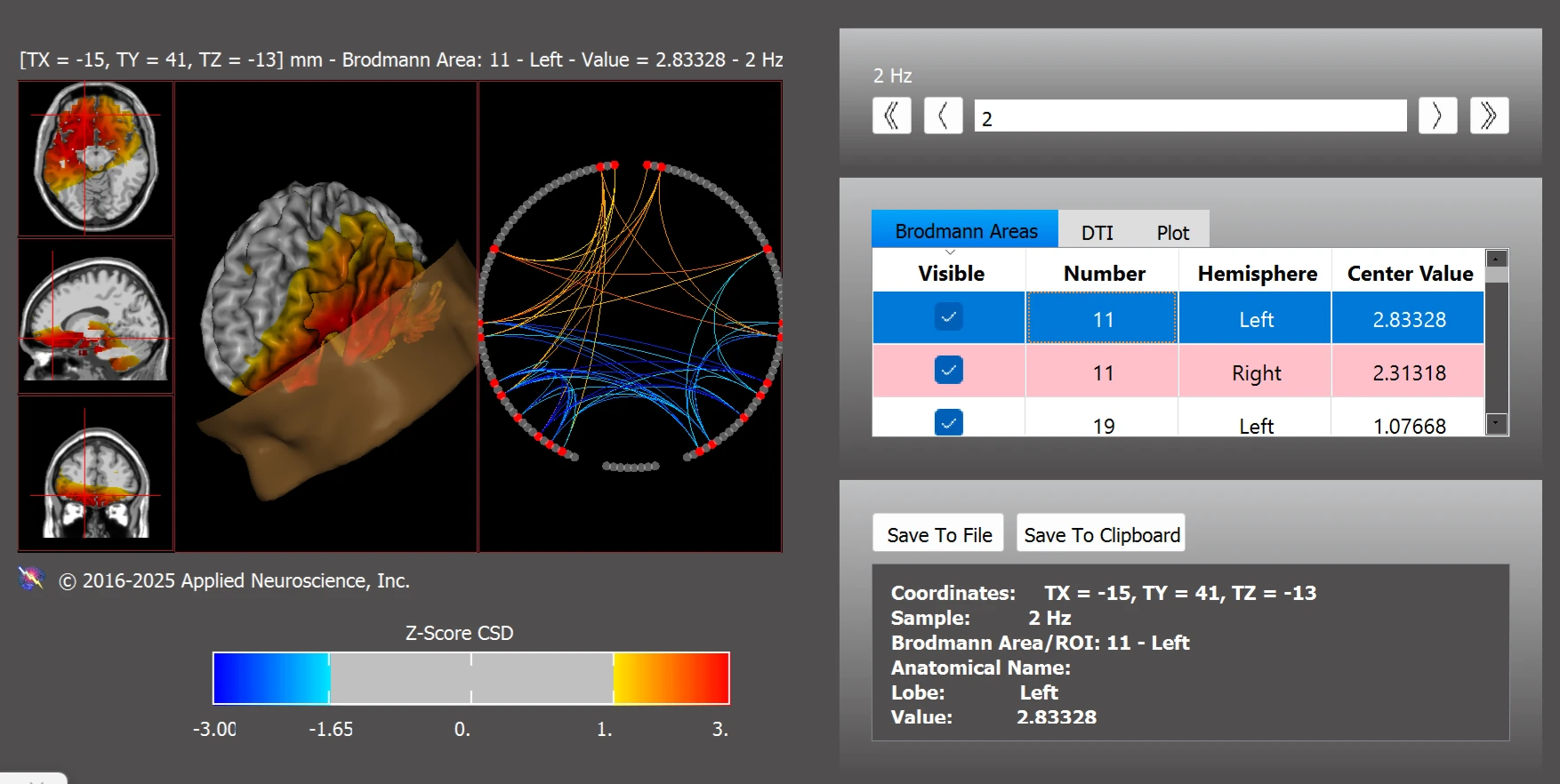
Note. Image shows z-score deviations in functional connectivity at 2 Hz within the default mode network. Data were analyzed using the NeuroNavigator module in NeuroGuide.
Dysregulation was also observed within circuits associated with reward processing with notable z-score deviations in BAs 13aL, 13aR, 25L, 25R, 34L, 34R, 47L, 47R, as well as subcortical structures including the left and right thalamus, habenula, amygdala, and nucleus accumbens (see Figure 4).
Figure 4. Functional Connectivity Within the Addiction/Reward Network
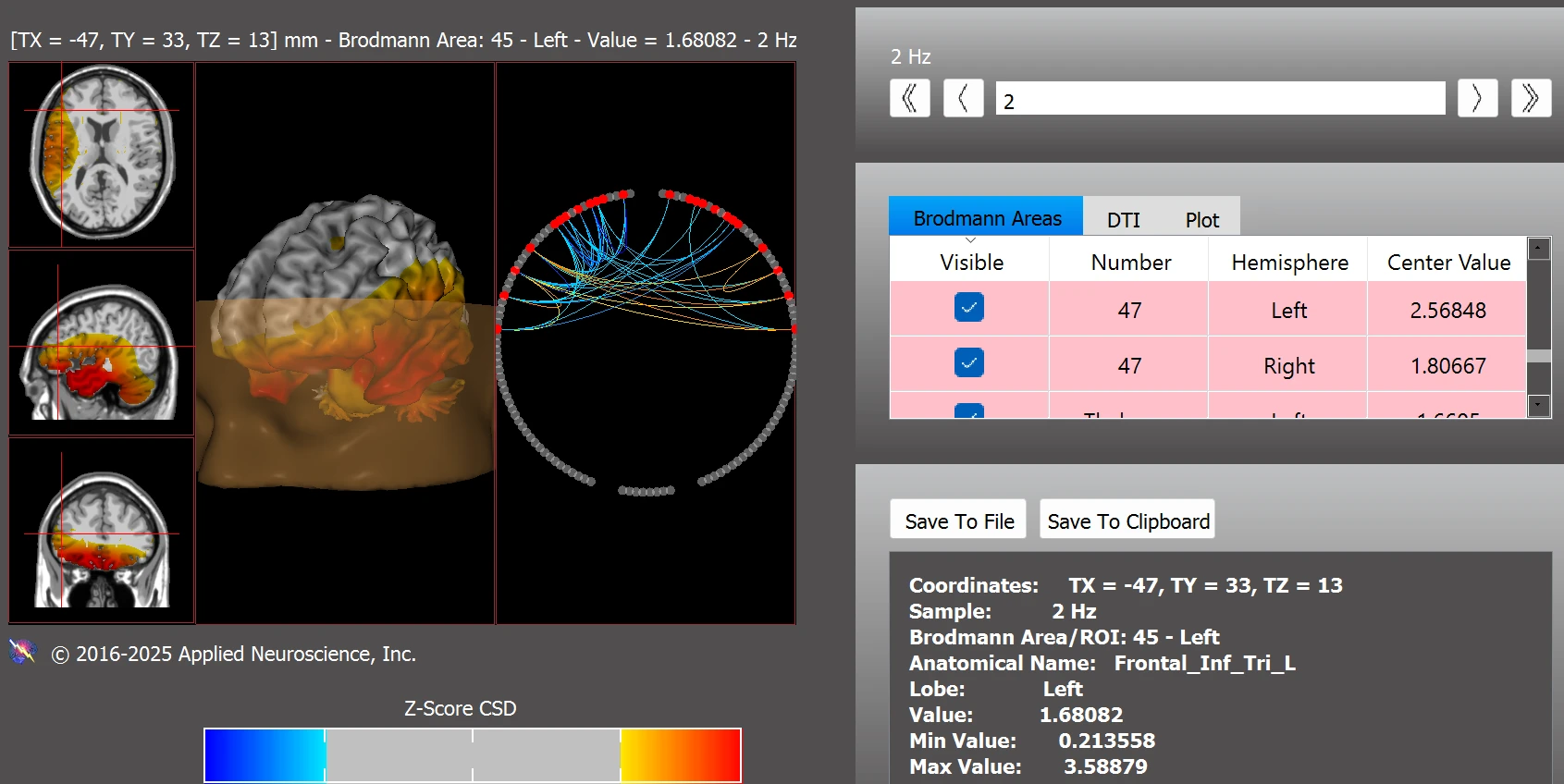
Note. Abnormal connectivity in reward-related regions including BA13, BA25, the thalamus, habenula, and nucleus accumbens. Z-score analysis conducted using NeuroNavigator.
In addition, Leo’s connectivity profile included patterns frequently reported in individuals on the autism spectrum with atypical activity in BAs 11L, 11R, 13aL, 13aR, 13pL, 13pR, 21L, 22L, 30L, 45L, as well as deviations in the cerebellum (left and right), vermis (left and right), and bilateral amygdala (see Figure 5).
Figure 5. Functional Connectivity Within the Autism Spectrum Disorder Network
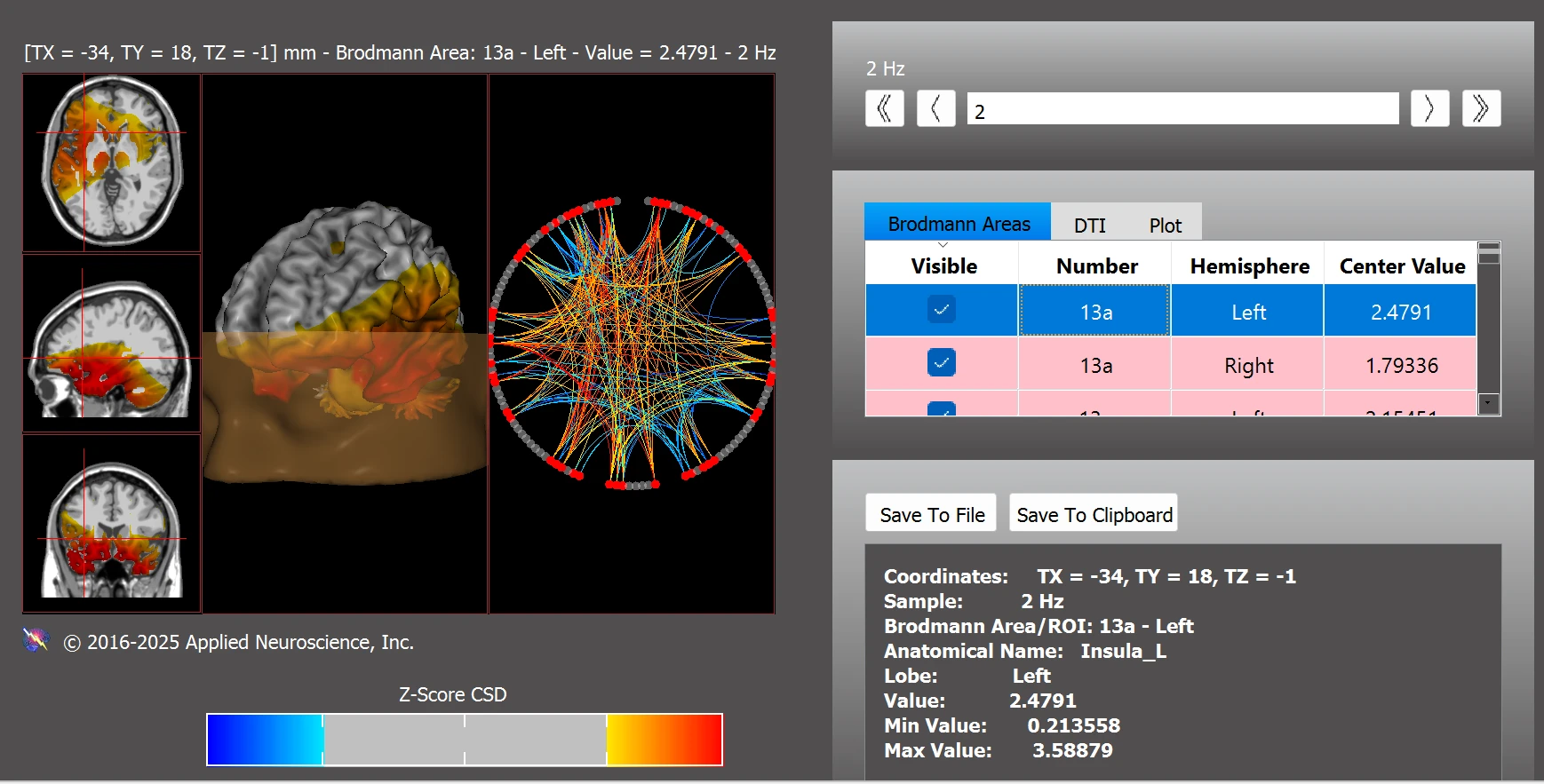
Note. Deviations in ASD-related brain regions, including BAs 11, 13a/p, 21, 22, cerebellum, and amygdala, based on z-scores from NeuroNavigator.
Further dysregulations were observed within the executive function network, with elevated z-score deviations in BAs 11L, 11R, 22L, and 37L (see Figure 6).
Figure 6. Functional Connectivity Within the Executive Function Network
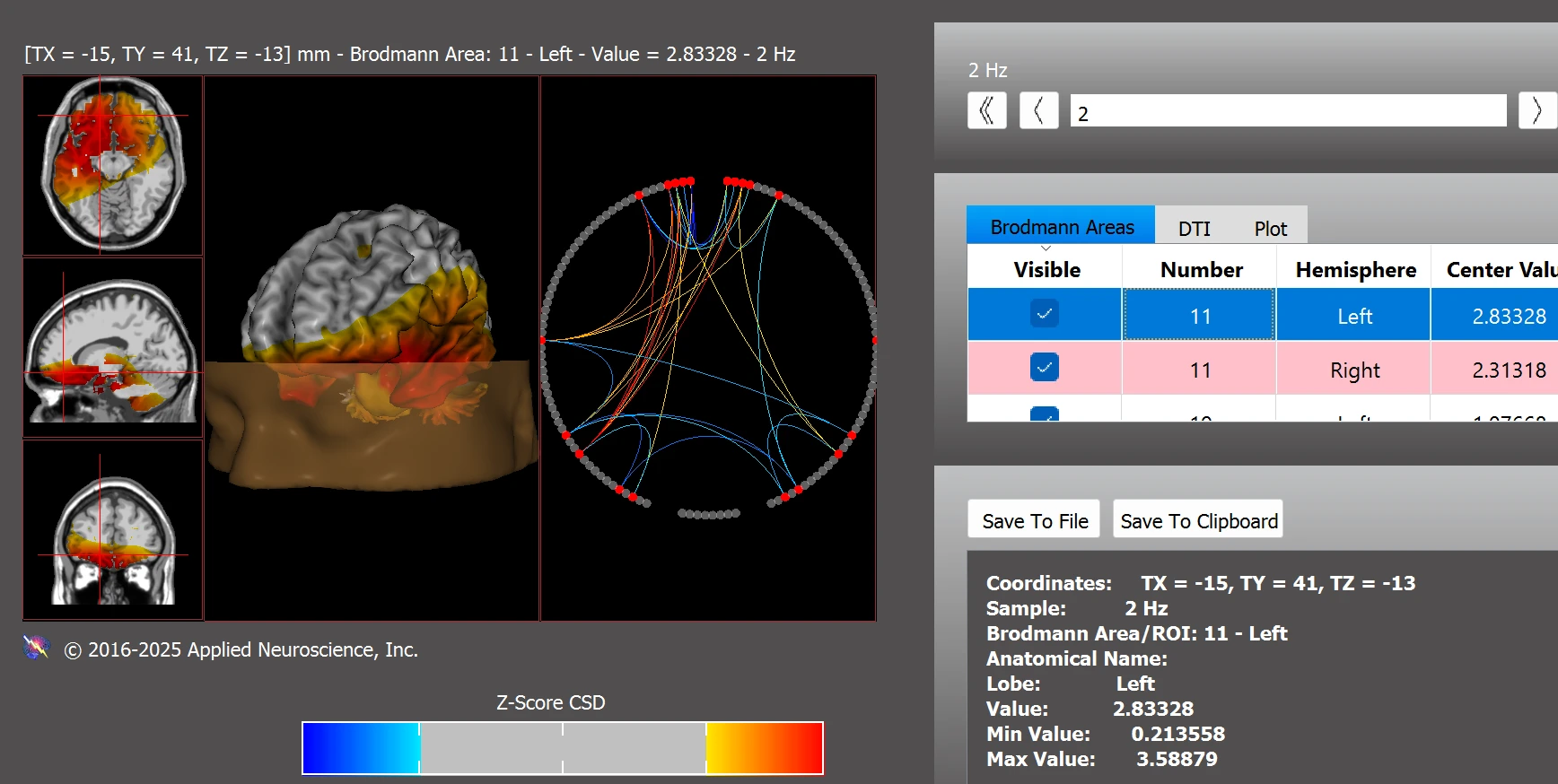
Note. Pre-training dysregulation in executive areas including BAs 11, 22, and 37. Data analyzed using NeuroNavigator.
Additionally, Leo exhibited abnormal functional connectivity in the amygdala, with z-scores indicating hyperconnectivity in both the right and left hemispheres (see Figure 7).
Figure 7. Right Amygdala (z = 2.98) and Left Amygdala (z = 3.49)
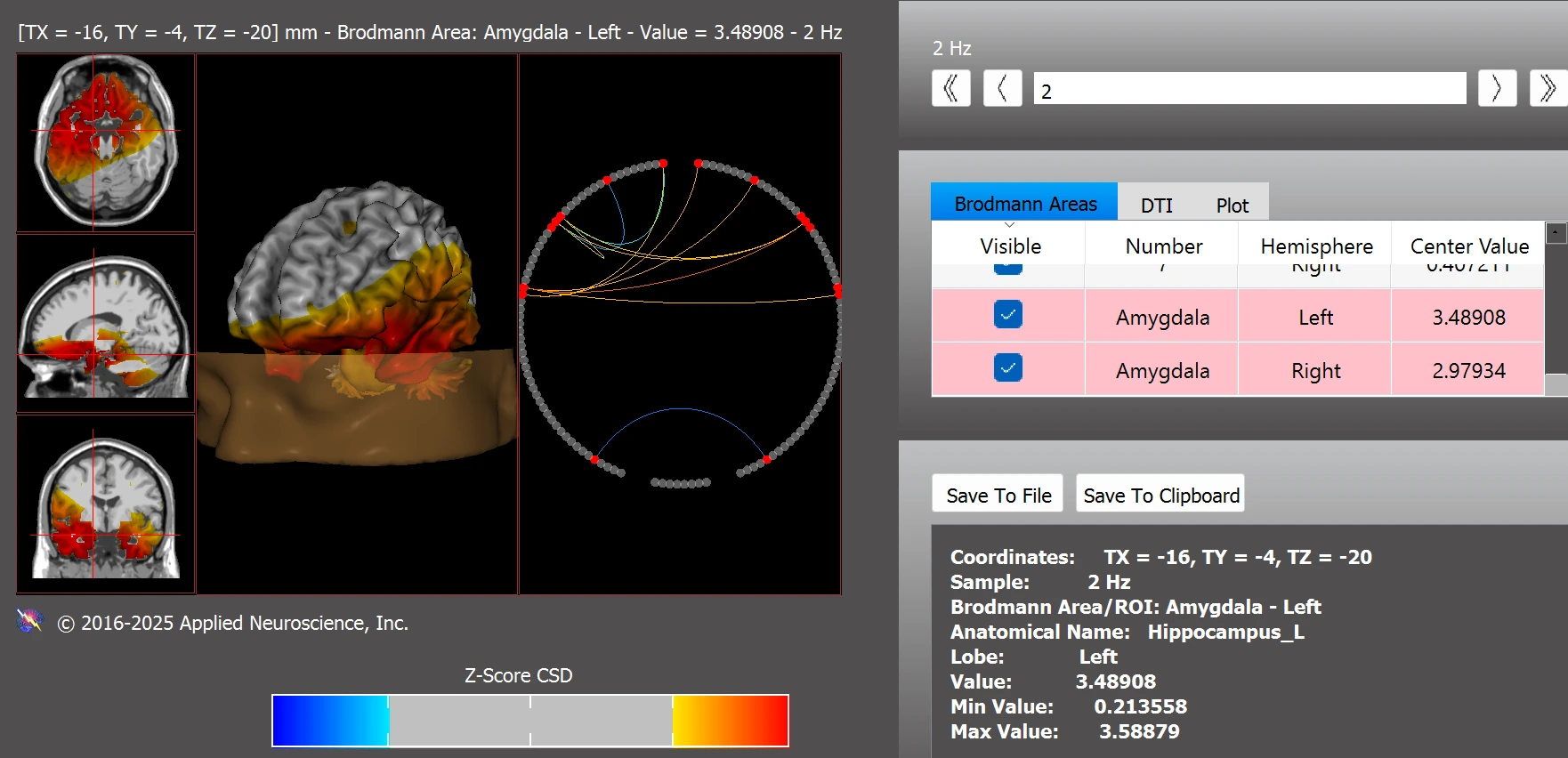
Note. Z-score deviations indicate hyperconnectivity in both the right and left amygdala. Data were analyzed using the NeuroNavigator module in NeuroGuide.
Neurofeedback Interventions
Leo completed 68 QEEG-guided surface neurofeedback sessions, each 30 minutes long, using BrainMaster Atlantis (BrainMaster Technologies, Inc., Bedford, OH). The training sites were based on the international 10-20 system. Initial training focused on downtraining excessive slow activity (1–5 Hz) at the left frontal and temporal sites (F7, T3, T5), followed by similar protocols at the right frontal and temporal sites (F8, T4). Later sessions inhibited high beta activity (25–29 Hz) at the posterior vertex (Pz), along with reinforcing alpha activity (8–12 Hz) to promote relaxation and cortical stability.
Throughout the intervention, Leo was supported by his family, whose consistent involvement contributed significantly to his participation and progress.
Results
Behaviorally, Leo became more alert, self-aware and responsive. According to his parents, several functional areas showed noticeable improvement after the neurofeedback intervention. His verbal abilities improved, and he was able to engage more meaningfully in communication with his parents. His speech increased substantially, broadening in vocabulary and becoming more pragmatic and less rote. In addition to these gains, his parents reported fewer tantrums and a noticeable reduction in emotional outbursts. These changes reflect improvements in emotional regulation, cognitive engagement, and social interaction—consistent with the neurophysiological findings observed post-training. Regarding device use, Leo prefers his phone but has also learned to navigate the TV, something he was unable to connect until after neurofeedback training, such as understanding the relationship between the remote control and the TV. As for screen time habits, his usage fluctuates; he tends to prefer screen time after a long day or when he needs a break from social interaction, indicating some adaptability in transitioning away from screens post-training.
Leo's post-training EEG was conducted in April 2025. The findings, shown in Figure 2 (right image), revealed the following improvements:
- Diminished delta activity at the prefrontal, frontal, and temporal regions.
- Reduced highbeta activity at the parietal and occipital areas.
- Diminished coherence within the delta frequency band.
In terms of functional connectivity, following the intervention Leo demonstrated improved integration within the default mode network (see Figure 8).
Figure 8. Functional Connectivity Within the Default Mode Network Post Training
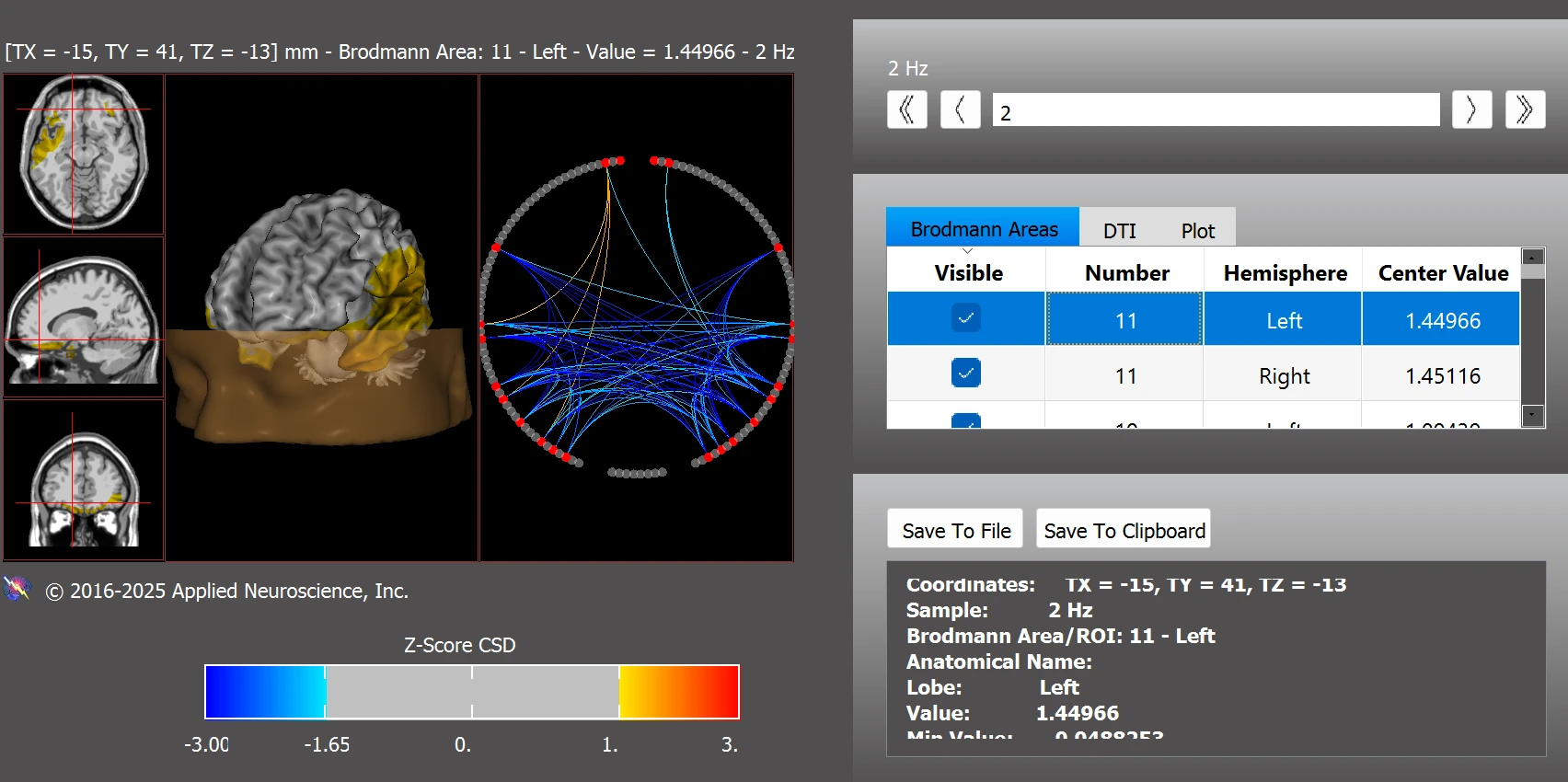
Note. Post-training z-score analysis indicates improved connectivity within the default mode network, with most regions returning to normative range. Data were analyzed using the NeuroNavigator module in NeuroGuide. Z-scores > |2.0| are considered clinically significant.
Additional improvements were also observed in reward-related circuits, with enhanced functional integration and reduced dysregulation following neurofeedback training (see Figure 9).
Figure 9. Functional Connectivity within the Addiction/Reward Network Post Training
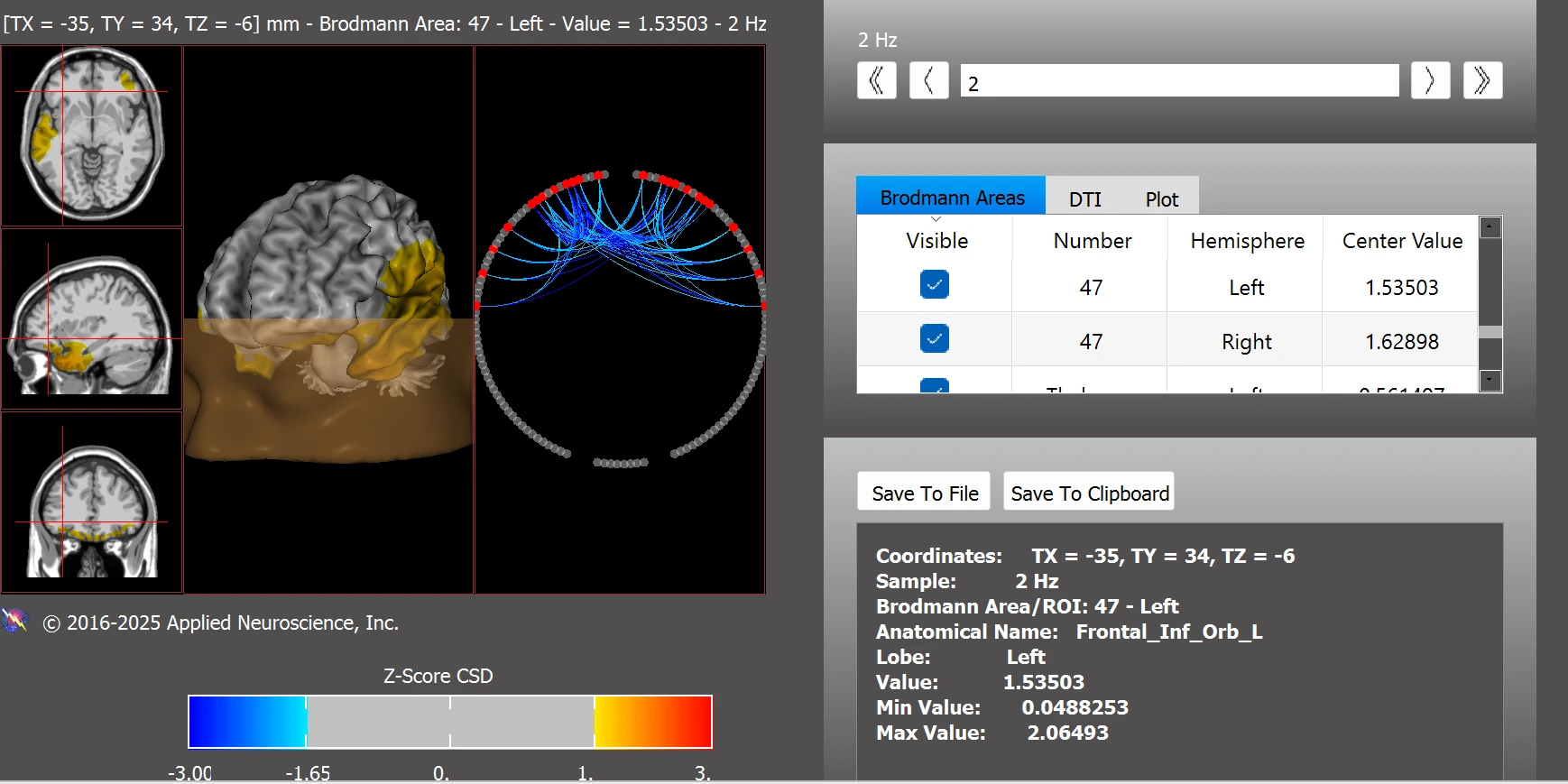
Note. Post-training functional connectivity analysis shows improved regulation within the addiction/reward network. Previously elevated z-scores in regions including BAs 13, BA25, thalamus, habenula, and nucleus accumbens were reduced toward normative levels. Data were analyzed using the NeuroNavigator module in NeuroGuide.
Improvements were also seen within the ASD network, with only minor post-training deviations remaining in BAs 13pL, 21L, and 22L (see Figure 10). These findings suggest substantial normalization of connectivity patterns associated with ASD-related neurophysiological profiles.
Figure 10. Functional Connectivity Within the Autism Spectrum Disorder Network Post Training
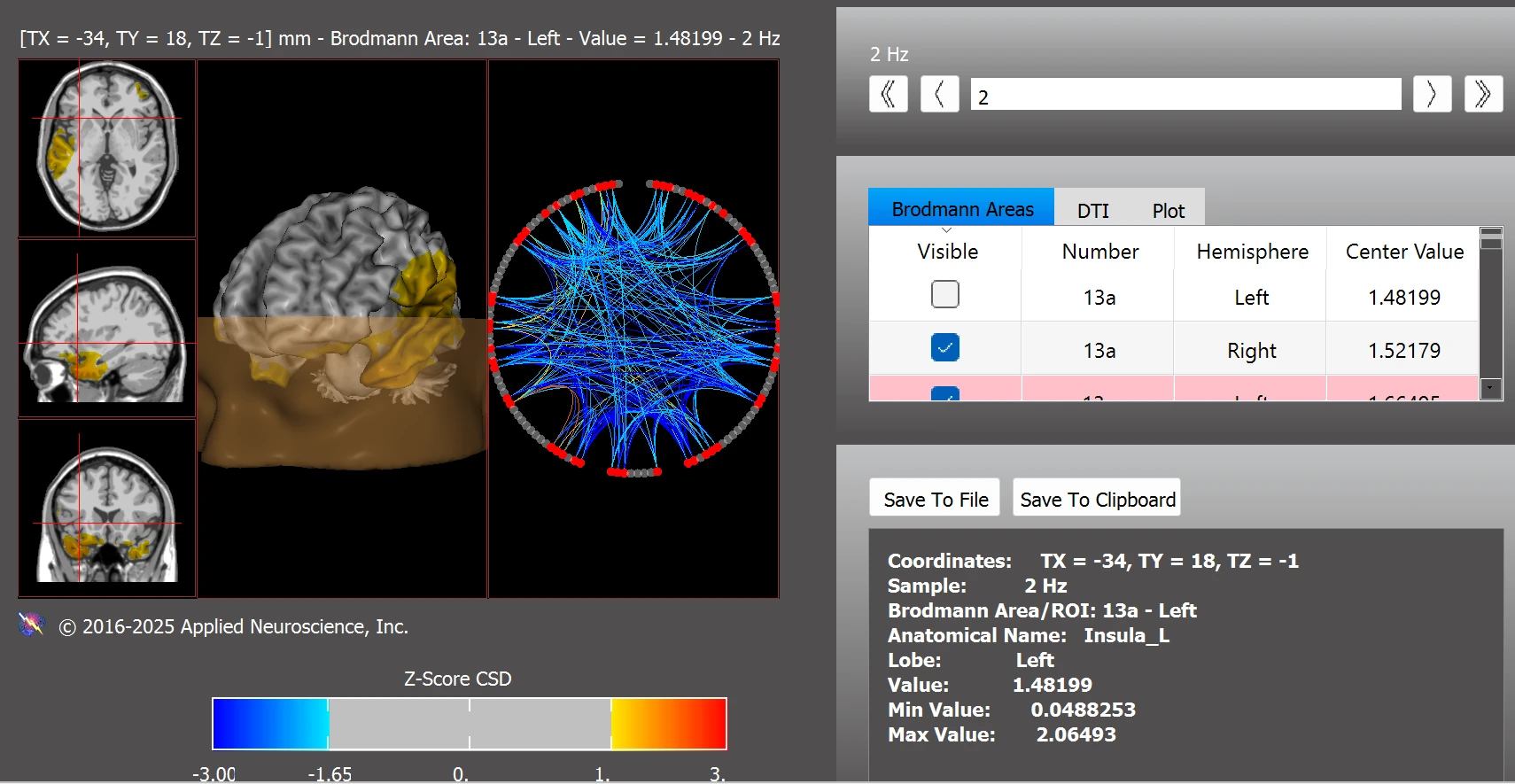
Note. Post-training z-score analysis reveals improved connectivity within ASD-related brain regions. Deviations remained only in BAs 13pL, 21L, and 22L, reflecting substantial normalization following neurofeedback. Data were analyzed using the NeuroNavigator module in NeuroGuide.
Post-training findings also indicated improved functional connectivity within the executive function network, with only a minor residual deviation observed in BA 22L (see Figure 11). This suggests enhanced frontal regulation and improved cognitive integration following neurofeedback.
Figure 11. Functional Connectivity Within the Executive Function Network Post Training
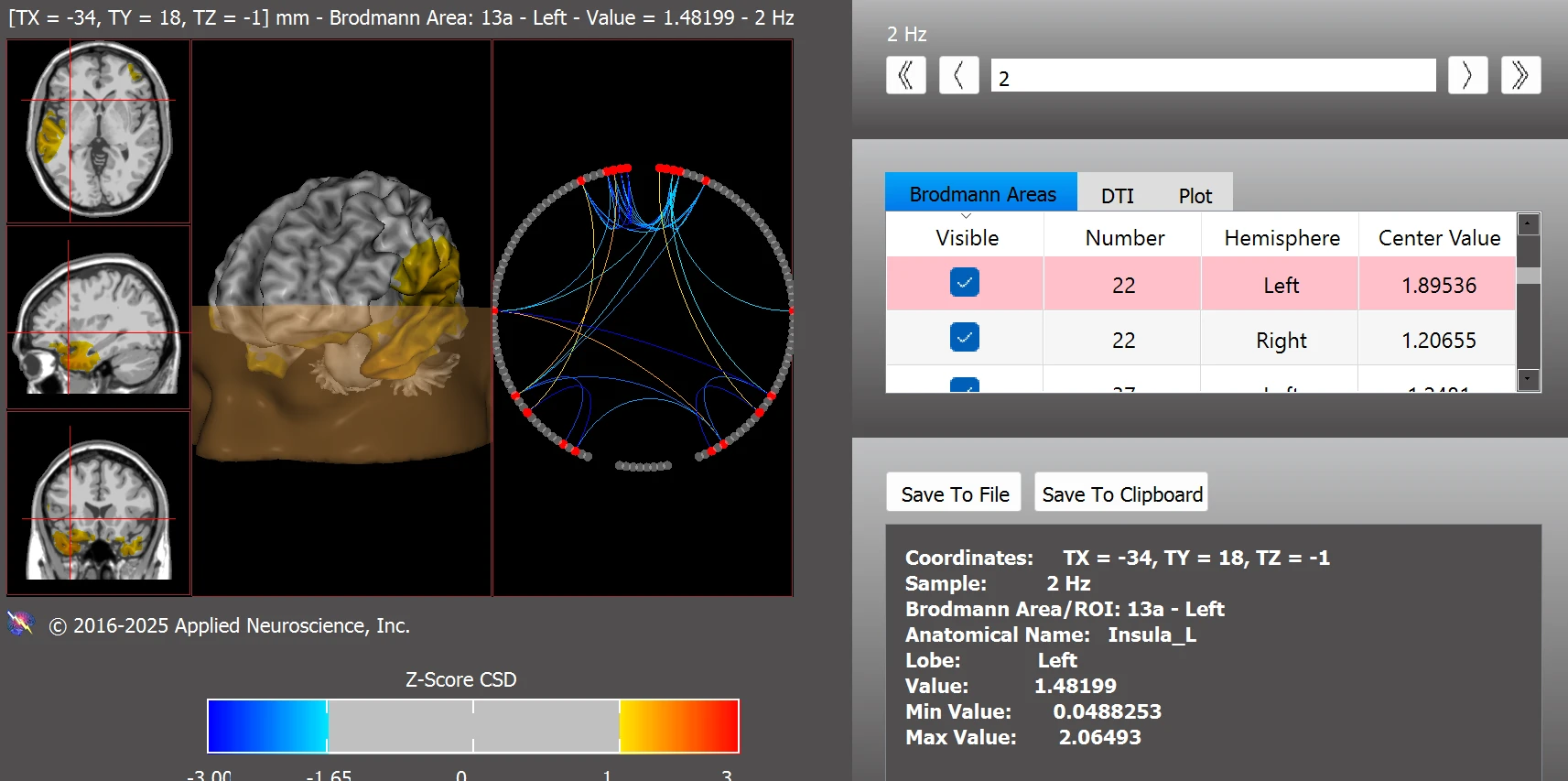
Note. Post-training analysis indicates improved connectivity within the executive function network. Remaining deviation was limited to BA 22L, suggesting enhanced frontal regulation and cognitive integration. Data were analyzed using the NeuroNavigator module in NeuroGuide.
Finally, the amygdala function showed notable improvement, with post-training z-scores indicating reduced hyperconnectivity in both the right and left hemispheres (see Figure 12). These changes may reflect improved emotional regulation and greater balance within the limbic system.
Figure 12. Right Amygdala (z = 1.39) and Left Amygdala (z = 1.63) Post Training
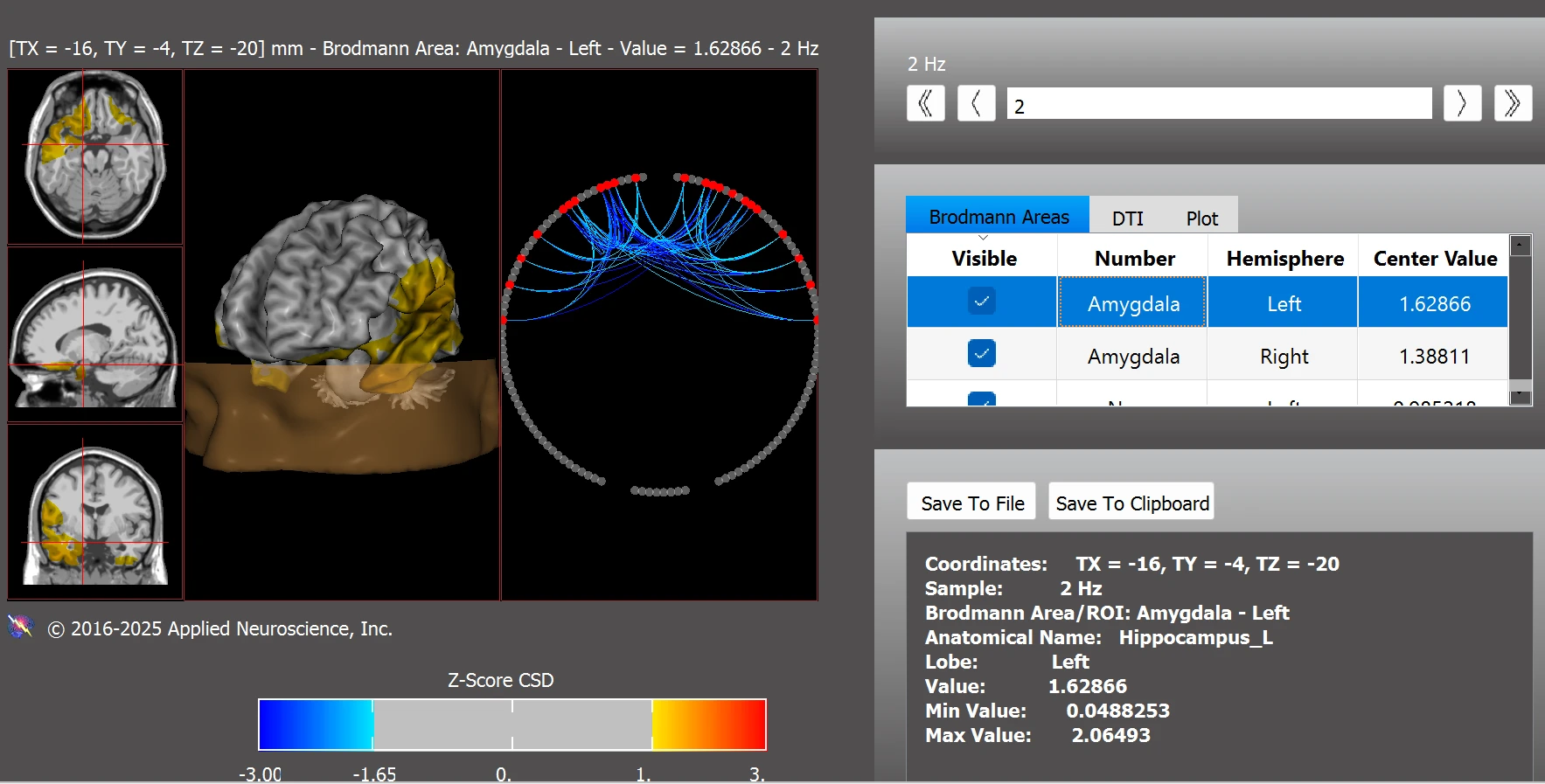
Note. Post-training z-scores show reduced hyperconnectivity in the bilateral amygdala, with values returning closer to the normative range. These improvements may reflect better emotional regulation and limbic system balance. Data were analyzed using the NeuroNavigator module in NeuroGuide.
Discussion
Collectively, these findings suggest that neurofeedback training can support positive neurophysiological changes and enhance self-regulation in children with ASD. Leo’s presenting symptoms, including under-arousal, apathy, limited speech, and emotional dysregulation, were reflected in his initial QEEG and functional connectivity findings. Dysregulated activity in Brodmann areas associated with emotion (BA11, BA13, BA25), language (BA21, BA22), and motivation (BA11, BA30, BA35) aligned with his behavioral profile. The neurofeedback training protocol targeted these regions indirectly through surface training and frequency-based modulation, resulting in marked improvement in both symptom presentation and neurophysiological measures.
In this case, the neurofeedback training may have helped modulate patterns potentially influenced by both developmental factors and environmental exposures, including screen use.
These results highlight the potential of neurofeedback as a non-invasive, individualized intervention for children with ASD, particularly those exhibiting screen-related dysregulation. Although this is a single case, the clear improvements in both clinical symptoms and brain activity show the benefit of using quantitative EEG to guide personalized neurofeedback training. The observed post-training normalization across multiple brain networks, including the default mode, reward, emotional, and executive function circuits, mirrors broader findings in the neurofeedback literature and supports its relevance in treating complex neurodevelopmental profiles. Future research should explore long-term outcomes, optimal training duration, and the interaction between neurofeedback and environmental modulation (e.g., screen time reduction, parental involvement) in larger samples.
Limitations
While current research highlights various negative associations between excessive screen use and neurophysiological alterations, it is important to acknowledge several limitations. Many cited studies are correlational, limiting the ability to infer causality between screen exposure and brain changes. Additionally, screen addiction lacks formal recognition in the DSM-5, resulting in variability in diagnostic criteria and assessment methods across studies. Individual differences such as genetics, environment, and co-occurring conditions may also be considered as factors. Future studies should use clear, consistent definitions and include diverse participants to enhance generalizability.
Conclusion
Excessive screen use during childhood and adolescence can profoundly affect neurodevelopment, particularly in brain regions involved in reward processing, executive function, emotional regulation, and habit formation. This overstimulation can decrease reward sensitivity, impair impulse control, and reduce attention and motivation. These findings highlight the importance of moderating screen use and encouraging interactive, non-digital experiences to support healthy brain development and psychological well-being in young populations. Mindful parenting practices have protective potential. Early childhood engagement in shared reading, outdoor play, and one-on-one interactions plays a critical role in shaping healthy brain architecture and fostering emotional resilience. Furthermore, neurofeedback as a neuroregulation tool shows promise in mitigating cognitive and emotional impairments associated with excessive screen exposure. Neurofeedback shows strong potential as a non-invasive, non-pharmaceutical intervention to support improvements in overall well-being, executive function recovery and emotional balance.
Acknowledgements
The author extends sincere gratitude to Dr. Robert W. Thatcher and the team at Applied Neuroscience, Inc. for granting access to the NeuroNavigator software for clinical analysis. Their support greatly enhanced the precision and depth of the QEEG and functional connectivity evaluation presented in this case study. The author also wishes to thank Leo’s family for their kind permission to use his de-identified information, which was essential for this research.
References
American Academy of Pediatrics. (2016). Media and young minds. Pediatrics, 138(5), e20162591. https://doi.org/10.1542/peds.2016-2591
Arns, M., de Ridder, S., Strehl, U., Breteler, M., & Coenen, A. (2009). Efficacy of neurofeedback treatment in ADHD: The effects on inattention, impulsivity, and hyperactivity: A meta-analysis. Clinical EEG and Neuroscience, 40(3), 180–189. https://doi.org/10.1177/155005940904000311
Centers for Disease Control and Prevention. (2023, October 30). Children's screen time. https://www.cdc.gov/ncbddd/screen-time/index.html
Chen, H., Dong, G., & Li, K. (2023). Overview on brain function enhancement of internet addicts through exercise intervention: Based on reward-execution-decision cycle. Frontiers in Psychiatry, 14, 1094583. https://doi.org/10.3389/fpsyt.2023.1094583
Chen, W., & Adler, J. L. (2019). Assessment of screen exposure in young children, 1997 to 2014. JAMA Pediatrics, 173(4), 391-393. https://doi.org/10.1001/jamapediatrics.2018.5546
Chen, Y. Y., Yim, H., & Lee, T. H. (2023). Negative impact of daily screen use on inhibitory control network in preadolescence: A two-year follow-up study. Developmental Cognitive Neuroscience, 60, 101218. https://doi.org/10.1016/j.dcn.2023.101218
Cheung, C. H. M., Bedford, R., Saez De Urabain, I. R., Karmiloff-Smith, A., & Smith, T. J. (2017). Daily touchscreen use in infants and toddlers is associated with reduced sleep and delayed sleep onset. Scientific Reports, 7, 46104. https://doi.org/10.1038/srep46104
Coben, R., & Myers, T. E. (2010). The relative efficacy of connectivity guided and symptom-based EEG biofeedback for autistic disorders. Applied Psychophysiology and Biofeedback, 35(1), 13–23. https://doi.org/10.1007/s10484-009-9102-5
Gray, P. (2011). The decline of play and the rise of psychopathology in children and adolescents. American Journal of Play, 3(4), 443–463.
Huang, P., Chan, S. Y., Ngoh, Z. M., Ong, Z. Y., Low, X. Z., Law, E. C., … Tan, A. P. (2024). Screen time, brain network development and socio-emotional competence in childhood: Moderation of associations by parent–child reading. Psychological Medicine, 54(9), 1992–2003. https://doi.org/10.1017/S0033291724000084
Hutton, J. S., Dudley, J., Horowitz-Kraus, T., DeWitt, T., & Holland, S. K. (2020). Associations between screen-based media use and brain white matter integrity in preschool-aged children. JAMA Pediatrics, 174(1), e193869. https://doi.org/10.1001/jamapediatrics.2019.3869
Koepp, M. J., Gunn, R. N., Lawrence, A. D., Cunningham, V. J., Dagher, A., Jones, T., Brooks, D. J., Bench, C. J., & Grasby, P. M. (1998). Evidence for striatal dopamine release during a video game. Nature, 393(6682), 266–268. https://doi.org/10.1038/30498
León Méndez, M., Padrón, I., Fumero, A., & Marrero, R. J. (2024). Effects of internet and smartphone addiction on cognitive control in adolescents and young adults: A systematic review of fMRI studies. Neuroscience and Biobehavioral Reviews, 159, 105572. https://doi.org/10.1016/j.neubiorev.2024.105572
Lou, L., Yan, W., Wang, L., & Zhang, Y. (2019). Altered default-mode network functional connectivity in college students with mobile phone addiction. International Journal of Clinical and Experimental Medicine, 12(2), 1731–1737. https://e-century.us/files/ijcem/12/2/ijcem0078031.pdf
Manolova, V. R., & Vezenkov, S. R. (2025). Screen trauma – specifics of the disorder and therapy in adults and children. Nootism, 1(1), 37–51. https://doi.org/10.64441/nootism.2NCSC.3
Mayo Clinic. (2023, April 21). Children and screen time: How to guide your child. https://www.mayoclinic.org/healthy-lifestyle/childrens-health/in-depth/screen-time/art-20047952
Nathanson, A. I. (2001). Mediation of children’s television viewing: Working toward conceptual clarity and common understanding. Annals of the International Communication Association, 25(1), 115–151. https://doi.org/10.1080/23808985.2001.11679002
Nie, Y., Pan, T., He, J., & Li, Y. (2025). Blunted neural response to real-life social reward anticipation in internet gaming disorder: An event-related potential study. International Journal of Psychophysiology, 207, 112479. https://doi.org/10.1016/j.ijpsycho.2024.112479
Nikken, P., & Jansz, J. (2006). Parental mediation of children’s videogame playing: a comparison of the reports by parents and children. Learning, Media and Technology, 31(2), 181–202. https://doi.org/10.1080/17439880600756803
Petrov, P. P., Dimova, V. R., Manolova, V. R., & Vezenkov, S. R. (2025). Screen time and policy approaches to digital media use in nurseries, kindergartens, and schools worldwide: A critical analysis. Nootism, 1(2), 41–50. https://doi.org/10.64441/nootism.1.2.4
Vezenkov, S. R., & Manolova, V. R. (2025). Neurobiology of autism/early screen addiction recovery. Nootism, 1(1), 19–36. (a) https://doi.org/10.64441/nootism.2NCSC.2
Vezenkov, S. R., & Manolova, V. R. (2025). Screen addiction – Biomarkers, developmental damage and recovery. Nootism, 1(1), 6–18. (b) https://doi.org/10.64441/nootism.2NCSC.1
Vezenkov, S. R., & Manolova, V. R. (2024). Rethinking autism: The screen addiction paradigm. Paper presented at the BFE 22nd Meeting, Ljubljana, Slovenia. https://doi.org/10.13140/RG.2.2.27970.80328
Volkow, N. D., Koob, G. F., McLellan, A. T., & Longo, D. L. (2016). Neurobiologic advances from the brain disease model of addiction. The New England Journal of Medicine, 374(4), 363–371. https://doi.org/10.1056/NEJMra1511480
Weisleder, A., & Fernald, A. (2013). Talking to children matters: Early language experience strengthens processing and builds vocabulary. Psychological Science, 24(11), 2143–2152. https://doi.org/10.1177/0956797613488145
Zenner, C., Herrnleben-Kurz, S., & Walach, H. (2014). Mindfulness-based interventions in schools-a systematic review and meta-analysis. Frontiers in Psychology, 5, 603. https://doi.org/10.3389/fpsyg.2014.00603