Violeta R. Manolova and Stoyan R. Vezenkov
Center for applied neuroscience Vezenkov, BG-1582 Sofia, e-mail: info@vezenkov.com
For citation: Manolova, V.R. and Vezenkov, S.R. (2025) Unified Trauma-Addiction Functioning Model. Nootism 1(4), 4-24, https://doi.org/10.64441/
Abstract
The Unified Trauma-Addiction Functioning (UTAF) Model reframes trauma, addiction, and psychosis as state expressions of the same nested autonomic hierarchy, whose healthy form is ventral vagal–led (VVNS) orchestration of sympathetic (SNS), dorsal vagal (DVNS), and enteric (ENS) platforms. Under chronic threat, disrupted co-regulation, or artificial overstimulation, regulation inverts into a mirrored “dark” hierarchy. Its universal gateway is the trauma–addiction loop, in which addictive functioning is not separate from trauma but an intrinsic aspect that creates, sustains, and complicates it over time. Here, the organism becomes dependent not on a specific substance or stimulus but on a state—an intrauterine-like, sensory-deprived safety experienced as “the winner takes it all.” Substances, cues, and compulsions are only vehicles for re-entry. This mode emerges when higher regulatory systems cannot resolve overwhelming stress, or when a stimulus triggers direct collapse into this evolutionarily older safety state.
In the dark hierarchy, mirrored VVNS uses nonhuman surrogates for co-regulation; SNS shifts to hyperarousal and defensive impulsivity; DVNS to dissociation and withdrawal; ENS to somatic dysregulation and psychotic disorganization. Early screen addiction exemplifies how compulsive surrogate use deepens trauma-related dysregulation, locking the nervous system in regression.
The therapeutic counter-model is VVNS-based self-regulation, offering absolute safety while keeping the organism awake, adaptive, and developmentally engaged. Thus, recovery requires restoring human attachment, fear-free connectedness, and cortical alertness in a relational context, progressively dismantling mirrored survival scaffolding. Healing is not erasing the dark hierarchy but reinstalling ventral leadership, enabling fluid movement among connection, mobilization, and rest, and replacing illusory safety with full participation in human life.
Keywords: Unified Trauma-Addiction Functioning (UTAF) Model; Polyvagal Theory; autonomic hierarchy; dark matryoshka; trauma–addiction loop; intrauterine regression; early screen addiction; psychosis; co-regulation; illusory safety mode
Introduction
Trauma and addiction are two topics of special importance to both researchers and therapists. Their impact is far-reaching, manifesting in a wide range of disorders, syndromes, and symptoms, and therefore accounting for a substantial part of therapeutic work. Each of these domains appears complex and multilayered, insufficiently studied to allow for consistently delivering indisputable relief, remission, and full recovery – further complicated by the patient’s age and life experience (with marked differences in etiology and symptomatology between children, adolescents, young adults, middle-aged, and older adults). Certain researchers and therapists have empirically observed that for many individuals, addiction and trauma are inseparably linked. The prevailing hypothesis is that addiction is preceded by trauma – whether a single traumatic event or chronic exposure such as toxic stress – and that receiving the “dose” (whether substance-based or behavioral) alleviates the pain and tension caused by dissociation and the inability to cope with the situation. This dynamic is reflected in numerous scientific and popular science works by leading researchers and therapists in the fields of trauma and addiction, whose extensive clinical experience and recognized scholarly reputation (e.g., Bessel van der Kolk, Stephen Porges, Peter Levine etc.) have shaped the modern understanding of the trauma–addiction relationship. Their contributions are rooted in physiological mechanisms active in the human body, offering a framework for interpreting the mental processes connected to these phenomena. The pragmatic value of this model – combining fundamental neurophysiological processes with the plasticity of learning and experience – provides a solid foundation, yet leaves room for refinement in areas where it cannot fully explain observed phenomena.
Stepping outside the boundaries of this established model, we introduce for discussion cases in which addiction appears before trauma symptoms. This is particularly evident in children under the age of three whose family dynamics are examined and show no signs of trauma during pregnancy, birth, or the postnatal period; there is no neglect, and parents demonstrate a good understanding of the child’s needs. Despite this, the child develops a severe addiction, and within months exhibits a spectrum of symptoms typical of a moderately to severely traumatized child. Similar patterns are observed in older children (10–12 years) and adolescents (14–16 years) who develop an addiction within a month or two, followed by a dramatic deterioration in their condition, again displaying hallmark signs of trauma. Investigation of these children and their families reveals no traumatic history, yet their functioning shows typical trauma markers. A comparable picture emerges in adults (26–35 years) who, over the course of a few months, develop a severe addiction that leads to a sharp decline in overall functioning, once again with evident trauma markers.
In all described cases, traumatic features manifest as physiological markers in the autonomic nervous system and cortical functioning, as well as in the individuals’ symptoms, reactions, and attitudes toward the world and themselves. However, these trauma-related patterns do not yield to therapeutic processing until the organism has been “cleansed” of the patterns of addictive functioning. This gives us two reasons to focus attention on addiction as a cause of trauma: first, because it induces functional changes identical to those seen in trauma; and second, because it becomes a critical obstacle in the recovery process. This leads to the central hypothesis of the present work - namely, that addiction can arise independently as a distinct mode of functioning, one that results in trauma with its characteristic symptoms and developmental deficits. In short: a person does not need to be traumatized beforehand to develop an addiction, but once an addiction emerges, it will lead to trauma; and once addictive functioning develops, trauma therapy remains hostage to the addiction.
There is literature showing that addiction can lead to trauma-like functioning in early screen exposure in young children (Marcelli et al., 2018; 2020). However, these works do not address the question of why addictive functioning is not rejected by the organism, is not recognized as threatening, and instead becomes generalized, over time forming a full trauma-like mode of functioning.
This gap in understanding opens space for a sub-hypothesis that enriches the primary one: addictive functioning can be understood as a coping strategy of the organism. It emerges alongside psychophysical dissociation, experienced as a powerful “disconnection” from the external world and a “closing off” into the internal world – specifically in terms of sensory experience. This dissociation may be triggered either by a traumatic event or by any form of stimulant, substance, or behavior that disrupts the natural balance of inward–outward sensory communication. In both cases, addictive functioning takes control over self-regulation, which in turn ensures the organism’s adaptive adjustment of vital processes to the demands of the environment.
The second sub-hypothesis draws a direct parallel between dissociation-linked addictive functioning and traumatic functioning, proposing that the severity of trauma depends on the duration of addictive functioning and on the extent to which it takes over specific regulatory systems, locking them into a collapse of maladaptive neuroception.
This paradigm assumes that addictive functioning forms when a child or adult experiences a dissociative reaction, the organism evaluates it as protective (beneficial), and over time it becomes generalized. The only way to prevent such a reaction from consolidating is to replace it with a more adaptive one - ideally, a healthy attachment pattern. However, when no adequate co-regulating significant person is available, the maladaptive reaction becomes the “winner takes it all.”
Children born with substance dependence due to in-utero exposure provide another striking example of primary addictive functioning without preceding postnatal trauma. Their dependence is physiological and present from birth (Irner, 2012). Clinical data indicate that these children also face significant developmental challenges, including a high risk of being diagnosed with ASD or ADHD (Sandtorv et al., 2018; Janecka et al., 2018). This underscores that neurobiological dysregulation associated with addiction – regardless of its origin (substance or overstimulation) - can lead to similar neurodevelopmental impairments. Numerous studies and meta-analyses have demonstrated that the developmental trajectory of these children, whether they receive a diagnosis or not, depends directly on the quality of the environment into which they are placed after birth (Bölte et al., 2019).
If the environment offers secure attachment, characterized by adequate parental care, high sensitivity to the child’s needs, and the capacity for co-regulation (i.e., caregivers who can provide a stable, nurturing, and responsive environment that activates the ventral vagal system), these children may avoid or significantly reduce the expression of neurodevelopmental disorders. Conversely, in the absence of such a supportive and co-regulating environment, they are extremely vulnerable to developing full-scale neurodevelopmental disorders, displaying the same symptoms as ASD and ADHD.
In the present paper, we examine children and adults with screen addiction as a distinct form of addiction linked to traumatic functioning. We aim to explain the mechanisms through which addictive functioning consolidates over time into traumatic functioning, and vice versa. The concept of screen trauma is proposed as a state in which screen addiction persists long enough to alter the organism’s functioning in a traumagenic manner. (Manolova & Vezenkov, 2025(1))
By framing addictive functioning as both a potential consequence and cause of trauma, we open the way for a more integrative model – one that recognizes a bidirectional, mutually reinforcing relationship between the two, underpinned by shared neurophysiological mechanisms. This expanded perspective allows us to approach phenomena such as screen addiction not simply as maladaptive behaviors (Vezenkov & Manolova, 2025 (4)), but as potential developmental pathways into trauma-related functioning.
In this study, we propose a new concept of traumatic–addictive functioning as a continuum, intended to serve as a unifying framework for both theoretical understanding and the development of integrated therapeutic interventions.
Bidirectional Pathways and Shared Mechanisms Linking Trauma, PTSD, and Addictive Disorders
Post-traumatic stress disorder (PTSD) and addictive disorders (including substance use disorders, SUDs, and behavioral addictions such as gambling or gaming) frequently co-occur and mutually aggravate one another. Clinical samples routinely show high comorbidity - often in the 30–60% range among people seeking SUD treatment—with comorbidity associated with more severe symptom profiles, higher craving, and poorer outcomes. (Houghton et al., 2023; Back et al., 2024)
Converging evidence suggests multiple bidirectional pathways: traumatic stress elevates risk for later substance or behavioral addictions (consistent with self-medication and stress-sensitization models), while heavy, dysregulated use increases exposure to new traumas and impairs recovery from existing PTSD. Recent longitudinal and meta-analytic work indicates PTSD predicts later drug-use disorder and harmful alcohol use via coping-motivated consumption and cue-triggered craving. (Amstadter et al., 2023; Luciano et al., 2022; Renaud et al., 2021)
At the mechanistic level, addiction and PTSD share partially overlapping neurocircuitry: chronic stress and trauma shift motivation systems from positive to negative reinforcement, recruiting the “dark side” of addiction (extended amygdala, anti-reward systems) while dampening executive control – dynamics also central to PTSD’s hyperarousal, avoidance, and dysregulation. This overlap may explain why trauma reminders can amplify substance craving and why trauma-focused care improves SUD outcomes when integrated. (Koob et al., 2013; 2016; Kwako & Koob, 2017; Wise & Koob, 2014) Systematic assessment of trauma history and PTSD is essential for all individuals with substance use disorder (SUD). Emerging evidence indicates that delaying PTSD treatment until full abstinence is reached may be unnecessary, as PTSD symptoms are directly associated with craving. This relationship strengthens the rationale for integrated therapeutic approaches, aiming to provide the most comprehensive and effective treatment outcomes (Renaud et al., 2021). Robust evidence indicates that PTSD symptoms - particularly hyperarousal, intrusive recollections, and negative affect - predict coping-motivated substance use, while trauma-related cues can directly intensify craving (Hawn et al., 2021).
Beyond DSM-style PTSD, ICD-11’s Complex PTSD (CPTSD) – which adds disturbances in self-organization (affect dysregulation, negative self-concept, relational disturbance) – maps closely onto addiction-related emotion dysregulation and interpersonal instability, and appears overrepresented in PTSD+SUD groups with histories of chronic/interpersonal trauma. (Cloitre, 2020; Karatzias et al., 2019; Patel et al., 2025)
PTSD symptoms have been linked to cognitive distortions in gambling and to problem gambling driven primarily by escape and avoidance motives. Emerging evidence also suggests that trauma exposure may increase vulnerability to problematic internet use and gaming, particularly among youth (Grubbs et al., 2018). Current narrative and methodological reviews consistently identify trauma as a significant risk factor for the development of gambling disorder and emphasize the need for more rigorous, causally oriented research designs in the field of behavioral addictions. (Alaba-Ekpo et al., 2024)
Gambling disorder is closely linked to childhood trauma, emotion dysregulation, and PTSD symptoms, with gambling often serving as a coping mechanism to escape distress. (Monson et al., 2023; Yao et al., 2025)
Trauma exposure is a robust transdiagnostic risk factor for behavioral addictions, often operating through PTSD and Complex PTSD pathways. (Levin et al., 2021)
A systematic review of 3,054 studies found limited and inconsistent evidence linking interpersonal trauma to later addictive behaviors, with positive associations more common for childhood than adult trauma. Most longitudinal research focused on alcohol abuse, rarely examined behavioral addictions, and often relied on retrospective trauma assessment, limiting causal conclusions. (Konkolÿ Thege et al., 2017)
A 2025 meta-analysis reports that childhood trauma significantly predicts internet addiction in adolescents with depression, with insomnia and alexithymia acting as mediators. (Imperatori et al., 2023; Wang et al., 2025)
A 2022 study found that adolescents with a history of childhood trauma were nearly twice as likely to develop internet addiction compared to their non-traumatized peers, with lower social support further amplifying this risk. (Sheng et al., 2022)
In a sample of Chinese college students, childhood trauma was significantly linked to Internet Gaming Disorder (IGD), with anxiety and depression jointly mediating this association. (Shi et al., 2020)
A 2024 study of Chinese students confirmed that the association between childhood trauma and IGD is partly mediated by depression, with psychological resilience moderating this pathway. (Liu et al., 2024) A 2025 structural equation modeling (SEM) analysis showed that childhood trauma directly contributes to social media addiction among university students, with emotion regulation difficulties serving as a key mediator. (Elkin et al., 2025) Compulsive shopping is fueled by self-regulation difficulties stemming from trauma, with modern digital shopping environments amplifying these behaviors. (Amani, 2023)
In their longitudinal community-based study, Haller and Chassin (2014) tested four theoretical models describing the relationship between trauma, PTSD, and substance use problems. The high-risk hypothesis proposes that substance use problems increase the likelihood of trauma exposure by placing individuals in dangerous situations or impairing their ability to detect and avoid danger cues. The susceptibility hypothesis suggests that substance use problems heighten vulnerability to developing PTSD symptoms following trauma, as they can undermine coping capacity, intensify physiological arousal, and promote avoidance. In contrast, the self-medication hypothesis holds that PTSD symptoms increase the risk of later substance use problems because individuals turn to alcohol or drugs to manage distressing symptoms such as hyperarousal, intrusive memories, and negative mood. Finally, the shared vulnerability hypothesis posits that PTSD and substance use problems arise from common underlying risk factors - such as family adversity or genetic predispositions - rather than from a direct causal relationship.
The results provided the strongest support for the self-medication hypothesis: PTSD symptoms significantly predicted higher levels of subsequent alcohol and drug problems, even after controlling for trauma exposure, pre-trauma substance use, family adversity, and demographics. Each additional PTSD symptom was associated with roughly a 10% increase in the risk of later substance-related problems. There was partial support for the high-risk hypothesis, as adolescent substance use problems did not significantly predict overall trauma exposure but did marginally increase the risk of assaultive violence, particularly in the context of binge drinking. The susceptibility hypothesis was not supported - once family adversity was taken into account, pre-trauma substance use problems did not predict PTSD symptom severity. Similarly, the shared vulnerability hypothesis was not supported; although family adversity increased the risk of trauma exposure and PTSD, it did not explain the association between PTSD and later substance use problems. Instead, PTSD symptoms mediated the effect of family adversity on subsequent alcohol and drug problems.
This pattern of findings highlights PTSD symptomatology as a key long-term risk factor for substance misuse and underscores the role of adverse family environments in indirectly increasing that risk by fostering vulnerability to PTSD.
María-Ríos and Morrow (2020) provide a comprehensive review of clinical and animal research aimed at understanding why post-traumatic stress disorder (PTSD) and substance use disorders (SUD) so frequently co-occur. They discuss multiple explanatory models - including exposure-related, self-medication, and shared vulnerability hypotheses - that offer different perspectives on this comorbidity. Central to their analysis is the identification of overlapping neurobiological mechanisms involving dopaminergic, adrenocorticotropic, GABAergic, and glutamatergic systems. Dysregulation within these systems affects emotional learning, stress responsiveness, reward processing, and inhibitory control, thereby increasing susceptibility to both disorders. The authors conclude that PTSD and SUD share common biological pathways, underscoring the need for integrated prevention and treatment approaches.
Screen Addiction and Screen Trauma
The harmful effects of screens on children are not a new concern. They were highlighted as early as 2011 by the French Association of Ambulatory Pediatrics (AFPA), repeatedly by the American Academy of Pediatrics (most recently in 2016), and by many others (Harlé & Desmurget, 2012). Long-term research, such as Linda Pagani’s study begun in 1998 and still ongoing, has repeatedly shown the worrisome impact of heavy television exposure on both children and adolescents - affecting attention span, school performance, and social relationships (Pagani et al., 2010; Simonato et al., 2018). In France, Serge Tisseron has consistently warned of screen dangers (notably through the “3-6-9-12” campaign). In 2013, the French Academy of Sciences issued an advisory (Bach et al., 2013) acknowledging both the benefits and risks of screens, though it contained little data on very young children and largely echoed earlier warnings.
More recently, research on young children has accelerated worldwide, with studies emerging from China (Wu et al., 2017), Romania (Zamfir, 2018), Germany (Poulain et al., 2018), Thailand (Chonchaiya, 2011), the United States (Kabali et al., 2018; Heffler et al., 2015; 2020; 2022), and Bulgaria (Vezenkov & Manolova, 2025(1); 2025(2); 2025(3); 2025(4); 2025(5)) among others. This list is far from exhaustive but illustrates that concern over early screen exposure is now global.
In the past few years, professionals from a wide range of fields – not only doctors, pediatricians, and child psychiatrists, but also psychologists, speech therapists, nurses, childcare assistants, kindergarten teachers, and nursery staff – have reported a growing frequency of troubling behaviors among children aged 6–8 months to 3–4 years.
The authors describe a cluster of clinical signs observed in young children with early and excessive exposure to screens of all types, which they propose to classify under the term “early and excessive screen exposure” (EPEE) syndrome. This syndrome is characterized by attention difficulties, language delay, fine motor impairment, an increasingly exclusive interest in screens, and relational problems such as aggression and instability.
In the most exposed children, signs can appear as early as 8–10 months and gradually consolidate during the second year of life. A distinctive feature of this syndrome is that symptoms often regress – or even disappear – if excessive exposure is stopped promptly. However, when such exposure continues beyond the age of 3–4 years, improvement tends to be only partial. The authors regard screens – particularly small, portable devices left for long periods in the hands of toddlers – as genuine neurodevelopmental disruptors.
Clinically, EPEE involves three main areas of disturbance: attention/concentration, language, and social relationships. Depending on the child’s age, symptoms may include:
- Communication and language delays (evident at 18–30 months), often preceded by reduced vocabulary, pseudo-language (e.g., echolalia of English words or numbers), or a mechanical prosody.
- An increasingly exclusive focus on screens at home.
- Little or no initiation of interaction with parents outside screen use, sometimes actively avoiding eye contact.
- Lack of interest in age-appropriate games, particularly construction or pretend play.
- Poor, repetitive spontaneous activities such as lining up toy cars or waving objects in front of the eyes.
- Difficulties engaging with peers (especially in older toddlers).
- Aggressive-like behaviors (throwing toys, tearing paper).
- Persistent agitation and inattention.
- Fine motor clumsiness in tasks like puzzles or shape-sorting, typically becoming evident around 18–20 months.
While not every child presents all symptoms, many display several of them. These patterns have been documented by numerous clinicians and illustrated in a 2017 video posted by A.-L. Ducanda, which shows typical presentations of EPEE in affected children.
Vezenkov and Manolova (2025 (4)) had argued that screen addiction – encompassing television, gaming, VR, and smartphones – had been greatly underestimated, particularly in the post-COVID-19 context, and had often co-occurred with disorders such as ASD, ADHD, and ODD without being recognized as a primary causative factor.
They had identified consistent neurophysiological signatures tied to age of onset, sex, and addiction duration, including altered EEG patterns (either slowed theta/alpha or accelerated beta activity), reversed hemispheric asymmetry, functional fragmentation across brain regions, and autonomic dysregulation suggesting cortical “shutdown” during screen use. Crucially, the dominant frequency at the onset of addiction had become imprinted – persisting into later developmental stages—creating a lasting developmental anchor.
Manolova & Vezenkov (2025)(1) have introduced and explored screen trauma as a distinct, pathologically persistent condition that arises from prolonged screen addiction and extends well beyond cognitive or behavioral addiction. Using qEEG and autonomic nervous system (ANS) metrics, the authors have differentiated individuals into four groups - those with neither addiction nor trauma, those with only screen addiction, those with only screen trauma, and those presenting both. Screen trauma has manifested in cortical fragmentation, autonomic dysregulation, and rigid, maladaptive brain functioning that could not be reversed by simple screen detox alone. Importantly, the study highlights that children are particularly vulnerable, developing screen trauma more rapidly than adults, and that effective recovery requires an integrated complex family therapy – targeting cognitive flexibility, emotional regulation, and autonomic balance in child and parents – rather than detox in isolation.
In children with early screen addiction, EEG recordings had revealed a distinct imprint in the dominant frequency that had been characteristic of the age at which the addiction had developed, and this imprint had persisted into later developmental stages, even if other frequencies had emerged. This imprint had formed the basis of the traumatic functioning observed in children who had shown developmental delays or a “developmental anchor.” (Vezenkov & Manolova, 2025 (2); (4))
Severe neglect, and even the deliberate harming of a child’s health by parents, constitutes a profound trauma for the child. In the modern digital era, we are witnessing a new form of Munchausen Syndrome by Proxy (MSbP) by identifying a more insidious type of caregiver-induced harm - chronic screen overstimulation ending with addiction and trauma - that can trigger developmental delays and neuroregulatory dysfunction in children diagnosed with, or showing traits of, autism spectrum disorder (ASD) and/or ADHD. (Manolova et al., 2025)
Neurophysiological Mechanisms in the Onset and Maintenance of Screen Trauma
Early, intensive exposure to uniform, high-intensity audiovisual stimulation—typical of digital media during critical developmental periods - can reconfigure sensory–motor systems and prediction circuits in ways that mimic core features of autism spectrum disorder (ASD) yet remain environmentally induced and reversible. Clinical observation and targeted intervention have identified three interlinked pathological patterns:
- Screen-Induced Pathological Vestibular Reflex (SIPVR) – a maladaptive vestibular reaction in which inversion of the body triggers primitive reflex activation, postural collapse, and acute fear responses, absent in typical development. (Vezenkov&Manolova, 2025(5))
- Screen-Induced Pathological Eye-Covering Reflex (SIPECR) – a unique panic response to having the eyes covered, characterized by intense distress and loss of behavioral control; reliably present in affected children and abolished after complete screen detox, yet reactivated by screen-related cues. (Vezenkov&Manolova, 2025(3))
- Screen-Induced Synesthesia and Cue-Dependent Behavior – persistent cross-modal associations (e.g., visual–motor coupling, color–person mapping) and rigid behavioral dependencies in basic functions such as eating or sleeping, driven by overstimulation-induced dominance of audiovisual pathways and erosion of cognitive flexibility. (Manolova&Vezenkov, 2025(2))
These conditions share convergent neurobiological features: disrupted vestibular–visual integration, deformation of predictive coding maps by repetitive audiovisual cueing, reactivation of primitive reflexes, and functional replacement of adaptive regulatory strategies with compulsive, state-bound responses. Crucially, all three are plastic phenomena: with structured intervention - combining complete screen withdrawal and sensorimotor reintegration - they can diminish or disappear, whereas re-exposure reliably reinstates them. (Vezenkov&Manolova, 2025(1); 2025(4)); Petrova et al., 2025)
The recognition of SIPVR, SIPECR, and screen-induced synesthetic/cue-dependent patterns as environmentally driven syndromes reframes them not as immutable traits but as maladaptive neurodevelopmental states with high recovery potential. This perspective underscores the need for refined diagnostic criteria to distinguish between primary neurodevelopmental disorders and secondary, screen-induced developmental trauma. (Manolova & Vezenkov, 2025(1); Vezenkov & Manolova, 2025(3); 2025(5))
These mechanisms represent only the first described manifestations of screen trauma, with many others likely awaiting discovery through future research.
Biofeedback and Neurofeedback in the Treatment of Trauma, PTSD, and Addiction
Kwako and Koob (2017) framed addiction as a progressively worsening three-stage cycle – binge/intoxication, withdrawal/negative affect, and preoccupation/anticipation—with stress playing a central role in the transition to compulsive substance use. In the withdrawal/negative affect stage, stress-related neurocircuitry – including systems involving corticotropin-releasing factor (CRF), norepinephrine, and dynorphin – is activated alongside a diminished reward system, reinforcing motivation for continued drug use via negative reinforcement. These overlapping neurobiological adaptations illustrate how chronic stress both mirrors and amplifies the neural changes produced by repetitive drug exposure, exacerbating craving and relapse risk. Recognizing the primacy of negative emotional states and stress in driving this stage, Kwako and Koob introduce the Addictions Neuroclinical Assessment (ANA), spotlighting measurable subdomains of emotional dysregulation for both human and animal research. By emphasizing stress – and particularly negative emotionality – as a critical lever in addiction's neurobiological progression, their framework shifts clinical focus toward personalized interventions targeting the stress-addiction interface.
The Peniston Protocol, developed by Peniston and Kulkosky, combines temperature biofeedback and alpha–theta EEG training to address addiction and trauma-related disorders. In their seminal clinical trial, Peniston and Kulkosky (1989) demonstrated that alcoholic patients undergoing this intervention exhibited increased alpha and theta brainwave activity, stable beta-endorphin levels, reduced depressive symptoms, and significantly lower relapse rates at 13-month follow-up compared to controls. Later, Peniston and Kulkosky (1999) detailed the protocol’s mechanisms, highlighting its role in emotional regulation, personality normalization, and long-term abstinence. From a clinical perspective, White (2008) emphasized its transformational capacity to unlock and integrate unconscious traumatic material, underscoring the importance of the therapeutic environment and client receptivity. Collectively, these works position the Peniston Protocol as an early, integrative neurofeedback approach that bridges neurophysiological change with deep psychotherapeutic processing in the treatment of addiction and PTSD.
Graap and Freides (1998) critically examined the empirical foundation of the Peniston alpha–theta EEG biofeedback protocol, questioning the methodological rigor of existing studies. They noted issues such as small sample sizes, lack of appropriate control groups, and limited replication, which constrain the strength of conclusions about the protocol’s efficacy. While acknowledging promising clinical reports, the authors emphasized the need for well-designed, controlled trials to establish the protocol’s validity for treating alcoholism and PTSD.
Brownback and Mason (1999) discussed the application of neurotherapy in treating dissociation, emphasizing its relevance for trauma-related disorders and complex PTSD. They described how quantitative EEG (qEEG) assessment can identify dysregulated brainwave patterns linked to dissociative symptoms, which can then be targeted through individualized neurofeedback protocols. The authors highlighted that restoring more adaptive neural activity can reduce dissociative episodes, improve emotional regulation, and enhance integration of traumatic memories.
Lima, Gomes, and Tucci (2022) provided a critical review of electroencephalographic (EEG) neurofeedback as a harm-reduction tool for alcohol use disorder (AUD). They reported that neurofeedback—particularly alpha–theta and sensorimotor rhythm (SMR) training—shows potential for reducing alcohol consumption, craving, and relapse risk, while also improving emotional regulation and cognitive performance.
Russo, Smith, and Sperandio (2023) conducted a meta-analysis evaluating the effectiveness of neurofeedback in treating substance use disorders (SUDs) across multiple protocols and study designs. Their findings indicated that neurofeedback interventions yielded small-to-moderate overall effect sizes in reducing substance use, with some protocols—particularly alpha–theta and SMR (sensorimotor rhythm) training - showing the most consistent benefits. Improvements were also noted in secondary outcomes such as craving reduction, mood stabilization, and cognitive functioning, suggesting broad therapeutic potential. However, the authors highlighted significant variability in methodological quality, with many studies lacking rigorous control conditions, standardized outcome measures, or long-term follow-up. They concluded that neurofeedback represents a promising adjunctive treatment for SUDs but emphasized the need for more robust randomized controlled trials to strengthen the evidence base and guide protocol optimization.
Russo and Novian (2014) reviewed neurofeedback protocols targeting post-traumatic stress disorder (PTSD) and alcoholism, focusing on empirical evidence and clinical applicability. They found that alpha–theta neurofeedback, as pioneered in the Peniston Protocol, consistently produced improvements in PTSD symptoms, emotional regulation, and long-term abstinence rates in alcohol-dependent populations. The review emphasized that such protocols may reduce hyperarousal, enhance relaxation, and facilitate access to traumatic memories in a safe and controlled manner. The authors also noted methodological limitations in existing studies, including small sample sizes and inconsistent follow-up data, calling for more rigorous, large-scale research. Overall, they concluded that neurofeedback holds promise as a non-invasive, adjunctive treatment for comorbid PTSD and alcoholism, warranting further controlled trials to optimize protocols and confirm efficacy.
The treatment of PTSD and addictions using the theta/alpha neurofeedback protocol further demonstrates the shared neurobiological mechanisms underlying both conditions.
Neurofeedback for substance abuse is a promising approach with potential for treating difficult and resistant addicts who have gone through the revolving door of treatment many times. Beyond that, it has the potential for helping us learn about fundamental mind/body interactions and connections and even understand some of the mysteries connecting traumatic life events and substance abuse. (Trocki, 2006)
This section has demonstrated that addiction, PTSD, and emerging behavioral conditions such as screen trauma share overlapping neurobiological mechanisms, particularly in stress-related circuits and dysregulated self-regulation systems. Evidence from neurofeedback research – most notably the Peniston alpha–theta protocol – has shown promise in recovering brain activity, reducing cravings, and facilitating trauma integration, though methodological limitations remain. Integrating these insights into clinical practice highlights the potential for cross-cutting interventions that target both stress-driven addictive cycles and trauma-related dysregulation, offering a unified framework for treatment.
Polyvagal theory, trauma and PTSD
Polyvagal Theory reframes the autonomic nervous system (ANS) as a hierarchical, state-dependent controller of behavior that is organized by evolutionary history and tuned by the brain’s implicit detection of safety and threat. It posits three phylogenetically ordered response strategies: (1) a myelinated ventral vagal system (“social engagement”) that supports calm states, connection, and flexible self-regulation; (2) sympathetic mobilization (fight/flight); and (3) an older unmyelinated dorsal vagal immobilization system (shutdown/conservation), each recruited depending on whether the organism neuroceptively detects safety, danger, or life threat (Porges, 1995, 2001, 2003, 2007, 2009, 2011, 2021, 2022, 2024).
The ventral vagal complex (VVC) originates in the nucleus ambiguus and exerts rapid inhibitory control of the heart – the so-called vagal brake – enabling calm vigilance and swift transitions between engagement and action. It is neuroanatomically integrated with special-visceral cranial nerves (V, VII, IX, X, XI), coordinating facial expression, gaze, prosody, and middle-ear muscles to support social communication – what Porges calls the social engagement system. When safety cues are present, VVC tone stabilizes cardiac rhythms and fosters connection; when safety cues are absent, the brake releases, revealing sympathetic mobilization, and under life threat the organism may drop into dorsal vagal immobilization (Porges, 2001, 2003, 2007, 2011).
Neuroception refers to the nervous system’s nonconscious appraisal of safety, danger, or life threat that gates which autonomic platform becomes available. Trauma, adversity, and chronic stress can bias neuroception toward false alarms, keeping individuals locked in mobilization or collapse and constricting access to social engagement. Porges’s “vagal paradox” highlights that high vagal influence is adaptive in safe contexts (social calm) yet, via older pathways, can manifest as bradycardia/immobility under life threat – two faces of “vagal” physiology differentiated by pathway and context (Porges, 1995, 2007; 2023).
Respiratory sinus arrhythmia (RSA) - the high-frequency component of heart-rate variability – is advanced as a noninvasive index of cardiac vagal (VVC) regulation, with caveats about methodology and interpretation (Porges, 2007). Beyond baseline RSA, phasic reactivity and recovery – how quickly the vagal brake withdraws and re-engages – index vagal efficiency and flexibility. Neonatal sleep-state studies linked vagal regulation to early self-regulatory capacities, extending the theory into developmental psychophysiology (Porges et al., 1999).
Because VVC co-controls laryngeal, pharyngeal, and middle-ear musculature, autonomic state affects vocal prosody and auditory filtering (tuning to human voice frequencies). Under threat, the system shifts toward low-frequency vigilance, degrading prosodic communication and making social cues harder to send and receive – fuel for a vicious cycle of disconnection (Porges & Lewis, 2010; Porges, 2001, 2003).
Polyvagal Theory has been extended to gastrointestinal physiology: threat-biased autonomic states can dysregulate vagal–enteric pathways, helping to explain the co-occurrence of traumatic stress with functional GI disorders and feeding disturbances across development (Kolacz, Kovacic, & Porges, 2019).
Infants develop autonomic regulation within dyads: contingent facial affect, voice prosody, touch, and predictable routines from caregivers scaffold the child’s VVC tone and shape neuroception of safety. Disruptions (e.g., parental stress, inconsistent care) bias the child toward mobilization/collapse; supportive co-regulation restores access to social engagement (Kolacz & Porges, 2024).
In PTSD, threat-biased neuroception and diminished vagal flexibility trap individuals in chronic hyperarousal or intermittent shutdown, intensifying intrusive memories, exaggerated startle responses, and sleep disturbances; recent longitudinal research has linked PTSD symptom trajectories to autonomic complaints, reinforcing a bidirectional, state-dependent model (Kolacz et al., 2025). In borderline personality disorder, persistent emotion dysregulation and unstable relationships are associated with low or restless vagal tone and rapid defensive state shifts that undermine the capacity for social engagement (Austin, Riniolo, & Porges, 2007). In autism, challenges in social communication, atypical auditory filtering, and sensory defensiveness are viewed as expressions of reduced access to the ventral vagal complex (VVC) platform and altered neuroception; Porges (2025) proposes “deconstructing autism” by systematically introducing safety cues and autonomic state regulation strategies to facilitate social connectedness.
In trauma-informed practice, the first intervention target is state: creating cues of safety (predictability, prosodic voice, eye contact paced to tolerance, supportive touch when appropriate, posture/breath that slows heart) to recruit VVC engagement before deeper exposure or insight work. Interventions that leverage breath-cardiac coupling, vocalization/prosody, rhythmic movement, and therapeutic alliance are theorized to up-train the vagal brake and recalibrate neuroception (Porges, 2022, 2024). In addiction and stress-related conditions, this framework helps explain why negative affect and threat physiology drive relapse and why co-regulating environments improve outcomes.
Operationalizing safety has been a priority: the Neuroception of Psychological Safety Scale (NPSS) was recently validated in a UK adult sample, offering a psychometric tool aligned with the theory’s central construct (Cogan et al., 2025). Porges (2025) synthesizes current status and future directions, emphasizing measurement refinement (e.g., task-based RSA reactivity), developmental timing, and integrative clinical trials.
Polyvagal Theory positions safety as a foundational neurobiological resource. By elucidating how autonomic state shapes perception, communication, and behavior – and demonstrating how co-regulation can reopen access to the social engagement system – it provides a unifying, clinically actionable framework for addressing trauma, addiction, developmental challenges, and broader mental- and physical-health conditions (Porges, 1995–2025).
Conceptually, PTSD can be understood as a chronic dysregulation of the autonomic “hierarchy” in which neuroception persistently over-detects danger and collapses access to ventral vagal engagement, first defaulting to sympathetic mobilization and, with exhaustion or inescapability, regressing into dorsal vagal immobilization. This dorsal mode - an evolutionarily older conservation strategy - presents clinically as numbness, slowed cognition, emotional blunting, depersonalization/derealization, freeze/tonic immobility, and episodic shutdown, often alternating with bursts of hyperarousal; the oscillation itself reflects a system stuck between incompatible survival priorities. Because dorsal vagal dominance reduces metabolic output and dampens afferent interoceptive signaling to the cortex, it degrades fine-grained social perception (prosody, gaze) and learning, reinforcing avoidance and the sense that connection is unsafe—thereby tightening the trauma loop. State-dependent memory further binds traumatic content to immobilized physiology, so cues that nudge the system toward collapse can reactivate intrusive material even in the absence of explicit threat. Low resting RSA, sluggish vagal brake re-engagement, and impaired recovery from challenge index this loss of vagal flexibility, while the “vagal paradox” explains why powerful vagal influences can either support calm engagement (ventral) or bradycardic shutdown (dorsal), depending on pathway and context. Therapeutically, movement out of dorsal immobilization is not a leap but a titrated ladder: gently increasing mobilization (breath shaping, rhythmic movement, orienting) until the system can tolerate sympathetic energy without spinning into panic, and then recruiting ventral vagal channels (prosody, eye–face engagement, safe touch, co-regulation) to re-anchor safety. Framed this way, PTSD is less a fixed disorder than a maladaptive autonomic habit: a protective regression that made sense under life threat, but now requires carefully scaffolded experiences of safety-in-action to re-open the social engagement platform and restore flexible, trauma-incongruent responding. (Porges, 1995–2025).
Screen Trauma: Sensory–Predictive Collapse and Dorsal Vagal Dissociation
In infants and young children (under 3 years old), whose ventral vagus is still immature, the capacity for self-regulation is severely limited, and they are entirely dependent on co-regulation with an adult to restore balance after stress. When a child in this critical developmental period is exposed to intense and continuous overstimulation from screens, the rapidly changing images, unnatural colors, and sounds inevitably overload the immature nervous system (Neophytou et al., 2021). The brain is programmed to respond to novelty as a potential threat, but in the absence of an opportunity for an adequate fight-or-flight response or co-regulation, it shifts into a state of freezing or dissociation (dorsal vagal activation), which outwardly may appear as “calmness” or a hypnotic stare. During passive interaction with a screen, co-regulation with an adult is severely disrupted or entirely absent. The child is “disconnected” from the possibility of mutual interaction with the parent-reciprocal gaze, facial expressions, voice, touch - which are vital for the development of the ventral vagus and social engagement.
In these conditions, the child instinctively seeks a method of self-soothing outside of interpersonal interaction. They become “attached” to external, unchanging, and predictable stimuli that offer an illusion of control and stability. This leads to the formation of compulsive behavior and represents a primary addictive functioning. Clinical observations show that children with early screen addiction demonstrate significant developmental delays, particularly in areas such as language, socio-emotional skills, and executive functions (Marcelli et al., 2018; 2020; Heffler et al., 2015; 2020; 2022; Vezenkov & Manolova, 2025(2); 2025(4)). The symptoms of these children often completely overlap with those of Autism Spectrum Disorder (ASD) and Attention-Deficit/Hyperactivity Disorder (ADHD) (Zamfir. 2018; Heffler et al., 2022; Stefanova et al., 2025; Vezenkov & Manolova, 2024; 2025(2); 2025 (4)).
This occurs because the addictive functioning, caused by overstimulation and lack of co-regulation, keeps the brain and nervous system locked in a non-learning mode of compulsive reactions to environmental changes. Instead of developing flexible, adaptive circuits for self-regulation and learning from a dynamic environment, the brain becomes fixated on repetitive, predictable stimuli, which hinders the formation of complex neurological networks necessary for social interaction and flexible thinking.
Clinical Practice – Interventions and Recovery from Addictive–Traumatic Functioning
In line with the presented model, clinical observations and preliminary data from the Center for Applied Neuroscience “Vezenkov” show that recovery from screen addiction - including early-onset screen addiction - is possible and can lead to the resolution of associated symptoms such as autistic traits, manifestations of ADHD, and generalized traumatic functioning/PTSD in individuals of various ages and with varying symptom severity. This approach is entirely body-oriented and grounded in the fundamental concept that addictive functioning creates compulsive cycles which hinder neuroplasticity and the learning capacity of the brain and nervous system, immersing the organism in a traumatic experience of reality characterized by rigidity and non-adaptiveness.
At the purely physical level, the intervention focuses on freeing the body from compulsions expressed as automatisms and maladaptive reflex activity; working toward sensory integration to address existing psycho-physical dissociation; and restoring mirror systems and secure attachment, which allow individuals to learn and co-regulate effectively with others - thus breaking the mechanism of dependency on external objects and internal self-stimulation. This process clears the brain and nervous system from the dependency state, making them available for adaptive responses to environmental changes. In the spirit of the paradigms of Stephen Porges, Peter Levine, and Bruce Perry, the goal is to restore the body’s, nervous system’s, and brain’s capacity to learn to perceive and respond adaptively to live environmental stimuli (Porges, 2011; Levine, 1997; Perry).
Two key requirements for achieving these results have been identified:
- Specialized therapist training – Therapists involved in the process undergo advanced preparation in biofeedback therapy and in developing high heart rate variability (HRV), impeccable autonomic functioning, and optimal cortical alertness. This enables them to serve as accurate models for “imprinting”/mirroring and co-regulation for children, while eliminating fear or stress responses in the therapists themselves - a basic prerequisite critical for recovery. Therapists apply biofeedback principles regardless of whether they work with children and adults using equipment-based or non-equipment-based methods.
- Parallel parental biofeedback therapy – Alongside therapy for children, parents undergo biofeedback training to improve their functioning toward the ideal model. The mirroring and imprinting of addictive–traumatic functioning from parents onto children is a common phenomenon (Manolova et al.). This ensures that children are embedded in an environment where they can co-regulate effectively, rather than reinforce traumatic patterns of insecure attachment.
This recovery model reveals the profound link between addictive and traumatic functioning in both children and adults. Once the organism is freed from the cycles of addiction, the signs and markers of traumatic functioning diminish, vitality and learning capacity are restored, and symptoms begin to fade - often leading to full recovery.
The classical view of dissociation as a detachment from the inner world - a loss of access to emotions, bodily signals, and identity - does not align with the neuro-sensory reality observed in children and adults with screen or behavioral addictions. In these conditions, there is not a loss of internal life, but rather an intensified, unfiltered, and dysfunctional activation of inner experiences, occurring in the absence of sensory engagement with the environment. Here, dissociation arises not from the “switching off” of the internal domain, but from a sensory distortion of external reality, where the over-dominance of vision (through screens) suppresses other sensory channels - vestibular, tactile, and interoceptive. As a result, the inner world becomes a closed-loop resonance without external calibration, leading to disorientation, affective overload, and an inability to self-regulate. Dissociation, therefore, must be reconceptualized not as a lack of inner experience but as an inability to achieve sensory integration, in which the internal and external domains cease to remain in functional relationship. This reframing challenges the foundations of contemporary psychopathology, which is largely based on trauma-centered models, and calls for a sensory - rather than purely psychodynamic - reassessment of dissociative states.
Sensory integration deficits induced by early and chronic screen exposure not only distort the subjective experience of reality but also reconfigure the very predictive and adaptive models through which the brain operates. Overstimulation from screen-based environments leads to the formation of sensory-bound expectations - automated predictions anchored in hyperstable visual and auditory patterns that are artificially consistent and predictable. These patterns create a fixed predictive framework (“screen-induced prediction”) in which the world appears linear, controllable, and non-threatening - a context free of sensory noise, bodily pressure, or emotional complexity. When the child or adult is reintroduced to the real environment - one that is incomplete, unpredictable, and multidimensional - a significant prediction error emerges: a mismatch between expected and actual input that cannot be compensated for through either bodily or cognitive regulation due to the absence of a well-developed multimodal sensory foundation. This generates not just confusion but a neuro-sensory collapse, in which every new piece of real-world information is experienced as both sensorily indigestible and hostile. In this context, dissociation is not a reaction to trauma but a byproduct of an inability to process prediction error under conditions of disrupted interoceptive and exteroceptive synchrony. The dominance of vision, the artificial linearity of screen environments, and the eroded capacity to interpret external signals produce a severe form of functional isolation—not because perception is absent, but because the brain’s predictive map no longer aligns with reality. This misalignment drives incomprehensible behavioral responses, sensory distortions (including synesthetic episodes), and a compulsive need to return to the predictable, artificial reality of the screen.
Screen-induced dissociation is not simply a matter of “disconnecting” from the world, but rather the result of a sensory-driven distortion of the brain’s predictive system, in which the dominance of the visual–auditory modality reconfigures its predictive coding architecture. Prolonged screen overstimulation amplifies visual–auditory dominance, triggering structural changes in the visual cortex—including accelerated maturation and reduced cortical thickness (Richter et al., 2024; Hutton et al., 2022). This fosters fixed visual–auditory representations that generate low-variance predictions - stable, linear, and “noise-free” versions of reality. This is the essence of the screen-induced prediction phenomenon: the brain comes to expect the world to be as predictable as a screen simulation and is ill-prepared to handle multisensory noise - tactile, vestibular, or interoceptive. When reintroduced into a real, unpredictable environment, a substantial prediction error is generated in early visual areas (e.g., V1 exhibits heightened responses to high-level visual surprise, Nigam et al., 2024). Without a robust multisensory foundation, the brain cannot resolve these errors, producing what can be described as a neuro-sensory collapse - marked by severe confusion, disorientation, and subsequent dissociation. This is dissociation of integration, not of content - not the absence of an inner world, but the inability to align it with an external sensory map.
In a subset of children with established screen addiction, a phenomenon emerges in which, even after the long-term removal of access to digital devices, there is no overt seeking of screens, yet the propensity for dissociative states - achieved through self-stimulation - persists and may even intensify. These children develop repetitive sensory strategies - such as spinning colorful objects in front of their eyes, engaging in rhythmic head movements, maintaining fixed visual gazes, and other forms of self-generated sensory dominance - that serve as internal substitutes for prior screen stimulation. This behavior is not merely a residual habit but reflects a deeply ingrained neurophysiological pattern in which the nervous system has been “trained” to achieve a regressive pseudo-calm by severing integration with external reality. In this context, the dissociative matrix, initially induced by an external stimulus (the screen), has become endogenized and can be activated without any screen trigger. The child is not seeking the device itself but the effect - a state of sensory overdosing coupled with detachment from the present and suppression of interoceptive anxiety. This gives rise to a condition that can be conceptualized as self-stimulated sensory–dissociative addiction - a neuroadaptive survival mechanism in which reality is experienced as threatening, and self-imposed isolation becomes the primary regulatory strategy.
Self-stimulatory behavior observed in children with screen addiction after the permanent cessation of digital device use can be conceptualized as an endogenous neurosensory equivalent of substance intake. Similar to individuals addicted to alcohol, nicotine, or other psychoactive substances, these children are not seeking the stimulus as an object (the screen) but rather the neurophysiological effect it once produced - a sensory-dominated, regressive state of numbing, detachment, and pseudo-calm. Rhythmic movements, fixed gazes, and repetitive bodily actions function as self-generated neurochemical interventions, activating dopaminergic, opioid, and GABAergic pathways in a manner analogous to an external dose. The behavior carries hallmarks of compulsivity, short-term self-regulation, and dependency-driven repetition, typical of addictive patterns, but without the need for an external substance - the adapted body itself generates the “dose” required to avoid engagement with an environment perceived as unpredictable, aggressive, or empty. In this way, self-stimulation is not a residual echo of prior screen use but its internal replication - a form of neurosensory self-addiction in which the bodily act becomes the primary mechanism for controlling affect, arousal, and identity. This distinction is critical to separate it from behavioral stereotypies in neurodevelopmental disorders, as it represents a dynamic, acquired neuroadaptation that follows the logic of addiction rather than an innate deficit.
Developmental regressions and the cortical fragmentation observed in children with screen addiction are not the result of innate neurodevelopmental deficits but represent an acquired neurofunctional disorganization arising from chronic sensory distortion and impaired predictive adaptation. Prolonged overstimulation of the visual–auditory system induces both structural and functional changes - including overdevelopment and accelerated maturation of the visual cortex combined with cortical thinning in other associative regions, as documented by Hutton et al. (2022). This imbalance between sensory and integrative modules leads to fragmentation of cortical architecture, where different brain regions operate in an unsynchronized rhythm.
As a result, the brain’s prediction system is recalibrated to generate fixed, hyperstable expectations based on the artificially linear and controllable environment of the screen. When the child is confronted with a real-world, unpredictable, multisensory environment, a massive prediction error emerges - one that cannot be compensated for due to the absence of a robust interoceptive, vestibular, and tactile foundation. This produces a state of neurosensory catastrophe, in which external signals are experienced as indigestible, hostile, or disorganizing.
In response, the child regresses to earlier sensorimotor patterns - rhythmic movements, visual fixations, and self-stimulation - that serve as an internal substitute for previous screen stimulation. This forms a state of self-stimulated sensory-dissociative dependence, in which the body itself reproduces the “dose” of neurosensory isolation necessary to avoid connection with reality. This is not a residual habit but an adaptive neurophysiological mechanism for self-regulation under conditions of sensory and predictive disintegration. In this context, developmental regressions reflect a collapse of sensorimotor and affective–cognitive coordination, while cortical fragmentation represents the uneven allocation of neural resources in service of regressive rather than integrative processes. Reality is experienced as mismatched, and bodily and social connectedness becomes intolerable. Thus, dissociation in these children should not be understood as a purely psychological defense mechanism, but as the manifestation of a sensory–predictive collapse in which the brain loses the capacity to bind the internal with the external, the present with the anticipated, and bodily action with affective content.
As emphasized in the Screen Trauma report (Manolova & Vezenkov, 2025(1)), the body does not operate with predictive models for long-term consequences; instead, it reacts to immediate threats to homeostasis, even when the “solution” leads to enduring functional degradation. Thus, screen addiction is not an end in itself but a neurophysiological avoidance mechanism, in which the organism sacrifices development, sensory integration, and affective connectedness to stabilize within an artificial but subjectively tolerable reality. The long-term outcome of this adaptation is screen trauma - a state of chronic functional fragmentation, affective flattening, bodily disintegration, and impaired capacity to form self-coherence, autonomy, and genuine engagement. What may begin as “harmless” regressive self-stimulation evolves into a structural reconfiguration of the link between sensory processing, prediction, and identity - transforming reality from a stable, connective, and instructive space into a source of disorientation and secondary alienation.
Unified Trauma-Addiction Functioning Model (UTAF Model)
The Matryoshka Hierarchy of Autonomic Regulation
In the Unified Traumatic–Addictive Functioning (UTAF) model, regulation is pictured as a stack of nested control regulatory systems of autonomic platforms that emerge in a developmentally ordered way and hand control down the stack when a problem cannot be solved at the current level. (Fig. 1) We refer to these concentric functional layers as “matryoshkas” (nested systems, analogous to Russian nesting dolls) to emphasize their hierarchical activation and regression patterns. The outer matryoshka represents the most recently evolved, most flexible regulator; the inner matryoshka are older, more reflexive survival programs. When the outer system is active it does not silence the inner ones – it orchestrates them. When it cannot resolve a challenge (insufficient cues of safety, excessive load), it cedes control to the layer beneath. This orderly hand-off is what we call regression to an earlier developmental stage.
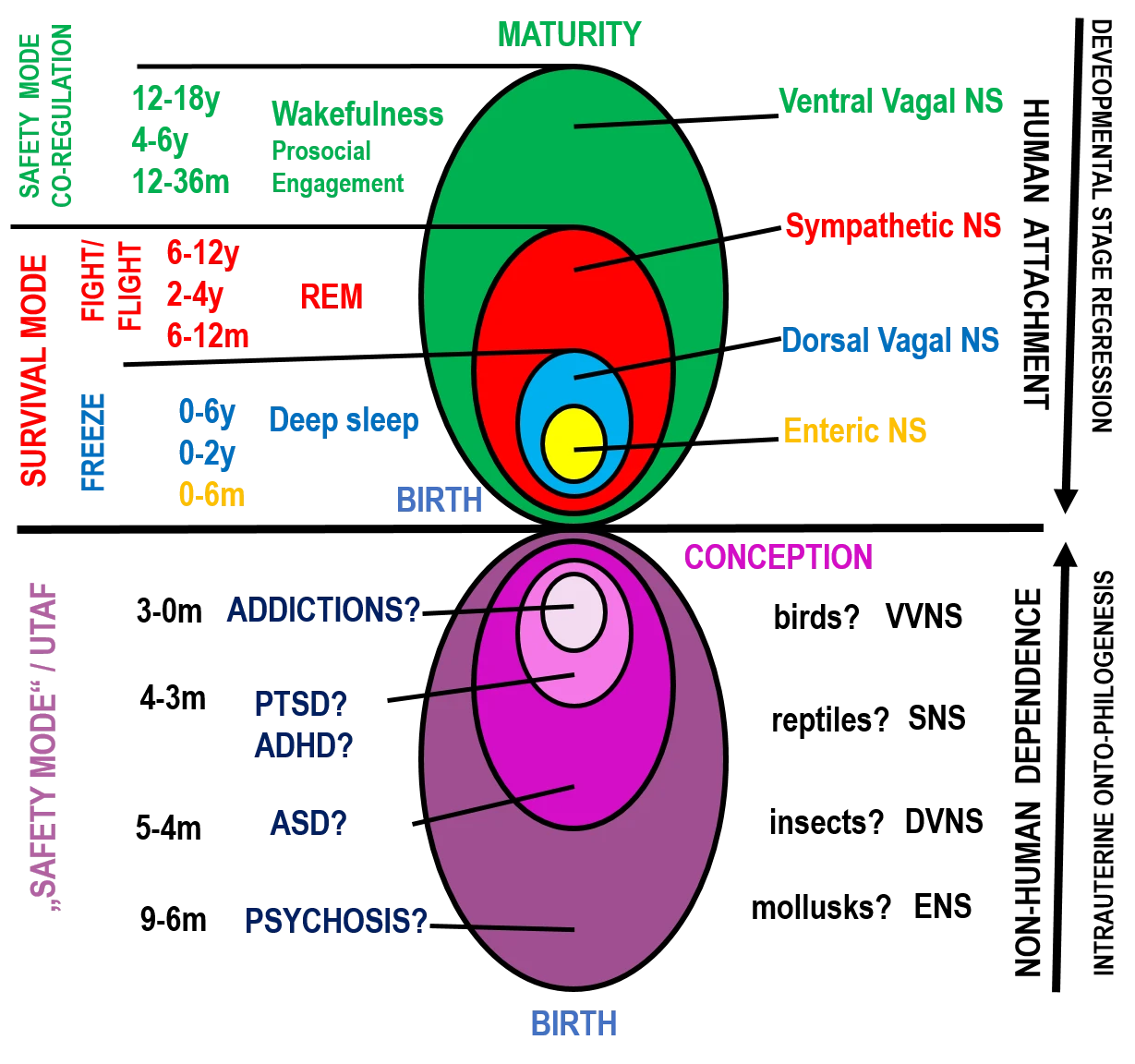
Figure 1. Healthy and mirrored (“dark”) matryoshka hierarchies of autonomic regulation in the UTAF model: from VVNS-led integration to inverted, illusory safety modes
The outermost layer is the Ventral Vagal Nervous System (VVNS), also called the social-engagement system. It applies the vagal brake to stabilize heart rate, supports calm vigilance, and synchronizes face, voice, gaze, and middle-ear function for social connection and learning. In development, VVNS is bootstrapped by co-regulation – caregivers lend their own physiological stability so that infants can borrow it. Across the timeline shown in the figure (major gains at 12–36 months, refinement at 4–6 years, integration at 12–18 years), VVNS becomes the conductor that keeps mobilization and immobilization available but well-timed, allowing swift shifts into action and smooth returns to rest.
Beneath VVNS sits the Sympathetic Nervous System (SNS) – the fight/flight mobilizer. SNS increases arousal, fuels muscle tone, sharpens focus, and drives goal-directed behavior. Milestones like crawling, standing, rule-based play, and organized sport (roughly 6–12 months, 2–4 years, 6–12 years) are periods when SNS’s capacities are strongly expressed – ideally under VVNS supervision. When safety-informed problem-solving fails, control hands down from VVNS to SNS, triggering mobilization. If the situation remains unsolvable or inescapable, the stack drops again.
The next inner layer is the Dorsal Vagal Nervous System (DVNS), which mediates immobilization – energy conservation, numbing, and analgesia. In early infancy (0–2 years) DVNS is a dominant substrate of state regulation; throughout life it supports deep non-REM sleep. In threat states, DVNS is the last-ditch survival strategy when mobilization fails. Crucially, DVNS is not “negative” by itself – when appropriately recruited (e.g., restorative deep sleep), it is essential.
At the core is the Enteric Nervous System (ENS) – the gut–metabolic baseline that orchestrates digestion and energy budgets. It is the earliest autonomic program (most active during 0–6 months) and remains the innermost support for all higher layers.
This stack also explains sleep-wake organization. In deep sleep, the upper matryoshkas are “diluted”: DVNS + ENS dominate while VVNS’s coordinating role is offline. In REM sleep, SNS surges (autonomic spikes, motor programs, vivid imagery) with limited top-down oversight. In wakefulness, all four systems – VVNS, SNS, DVNS, ENS – are active, but VVNS conducts the ensemble, enabling fast recruitment, clean disengagement, and recovery. Thus, resilience is the capacity to remain primarily in VVNS for flexible social engagement, to borrow SNS briefly and precisely for mobilization, to access DVNS for true rest and metabolic downshifting, to draw on ENS for stable energy and visceral homeostasis, and to return seamlessly to VVNS leadership – with all four systems active and integrated – without becoming stuck in any single layer.
The age bands in the figure indicate maturational inflection points for each layer. Early life is largely DVNS/ENS-led; mid-childhood showcases SNS under rules and play; late toddlerhood through adolescence installs and polishes VVNS leadership. In UTAF terms, health is the ability of the outer matryoshka (VVNS) to organize the inner systems. Regression – the hand-off down the stack – is adaptive in the moment; it becomes pathological when chronic threat, lack of co-regulation, or chronic stress or trauma keep the system pinned in SNS or DVNS.
The Dark Matryoshka: Addiction, Trauma, and Psychosis in the UTAF Model
When the organism is driven into survival mode, the path forward can branch in two fundamentally different directions. One leads back toward the human world: re-entry into reciprocal attachment, authentic co-regulation, and the reactivation of the VVNS as the conductor of the stack. This trajectory requires the presence of safety cues embedded in living interaction – gaze, voice, touch, shared affect – that restore the capacity for flexible engagement. The other path descends into the non-human world, where regulation is sought in the mirrored “dark” matryoshka hierarchy – an illusory safety mode in which stability is simulated through dependency on substances, objects, repetitive stimuli, rigid cues, compulsive behaviors, stereotypies, ritualistic acts, obsessive-compulsive patterns, catatonia, trance-like absorption, hallucinatory imagery, delusional constructs, synesthetic sensations, derealization, depersonalization, dissociation, thought blocking, or affective flattening. In this state, the nervous system clings to predictable but deceptive control, locking into maladaptive cycles that mimic safety yet block true integration. At its core lies the most “secure” state ever imprinted in all living, born organisms: the intrauterine life.
The regression along this “non-human” path may itself unfold in a matryoshka-like manner – layer by layer – yet its full structure remains to be mapped. The key distinction is that the same regulatory systems from the “human” hierarchy are still engaged here, but in distorted or mirrored form, operating in an entirely different context and serving survival through withdrawal from reality rather than engagement with it. For example, the mirrored VVNS manifests as the deep neurobiological drive underlying all forms of addiction – not as a platform for co-regulation and social safety, but as a compulsive search for predictable, controllable inputs that simulate connection while avoiding the risks of genuine human interaction. The mirrored SNS appears as the physiological substrate of chronic hyperarousal and defensive mobilization seen in PTSD, hypervigilance, ADHD-like restlessness, and impulse-control disorders. Progressively deeper layers in this mirrored descent may align with DVNS-based immobilization pathologies – such as severe dissociation, catatonia, affective flattening, or chronic withdrawal states – and ultimately reach ENS-dominated regressions marked by psychosomatic syndromes, severe metabolic shutdown, or primitive reflexive survival patterns.
555
In psychiatric terms, each mirrored layer carries its own phenomenology: the outer “addictive” VVNS-mirror fuels substance dependence, compulsive behaviors, object fixations, and pathological attachment to sensory cues; the mirrored SNS drives panic disorders, intermittent explosive behaviors, and intrusive re-experiencing phenomena; the mirrored DVNS anchors persistent dissociative states, depersonalization, derealization, psychomotor retardation, and even hallucinatory absorption; the deepest mirrored ENS regressions may correspond to psychotic disorganization, somatic delusions, or vegetative withdrawal that collapses both social and sensory engagement.
What does the UTAF model offer in this context? It identifies the precise functional level at which regulation has been hijacked into an illusory safety mode – thereby revealing the “entry point” for therapeutic work. By conceptualizing both adaptive and maladaptive patterns within the same nested architecture, the model reframes addiction, trauma, and psychosis as different manifestations of the same hierarchical control system under divergent environmental pressures. Resolving UTAF in its active layer is the prerequisite for safely “touching” and reintegrating the deeper regressions – those embedded in the mirrored, non-human hierarchy – without triggering further collapse. The therapeutic strategies and phased interventions derived from this framework will be presented in forthcoming publications, offering a stepwise approach to restoring the orchestration of all matryoshkas under VVNS leadership.
UTAF: The Key to the Dark Matryoshka
The Unified Traumatic–Addictive Functioning (UTAF) model is the central key to understanding the “dark” matryoshka hierarchy and its inner layers. It shows how the very same regulatory systems that sustain human attachment and safety can, under different conditions, become distorted into non-human, illusory safety modes. In this view, trauma, addiction, and even psychosis are not separate pathologies but different faces of one hierarchical state machine - built from the same autonomic, predictive, and learning systems, but operating in mirrored, maladaptive contexts.
In the healthy (“human”) hierarchy, the outer matryoshka—the Ventral Vagal Nervous System (VVNS) - leads, coordinating the Sympathetic Nervous System (SNS), the Dorsal Vagal Nervous System (DVNS), and the Enteric Nervous System (ENS). This orchestration allows flexible shifts between mobilization, rest, and metabolic regulation. In the mirrored (“dark”) hierarchy, each layer is still active, but its purpose is inverted: VVNS’s social engagement becomes compulsive attachment to substances, objects, or cues; SNS’s mobilization becomes hyperarousal, reactivity, and defensive impulsivity; DVNS’s restorative shutdown becomes pathological withdrawal or dissociation; and ENS’s metabolic base becomes the substrate for somatic delusions, vegetative regression, or psychotic disorganization. In the mirrored hierarchy, this represents a complete inversion of the normal matryoshka order - what is outermost in the human hierarchy (VVNS) becomes the innermost “core” of the dark matryoshka, wrapped by progressively older survival programs that now function as its protective shell, sealing the system inward rather than opening it outward to the world. The outermost layer of the dark matryoshka, now the mirrored ENS, serves not as a quiet metabolic foundation but as a rigid, somatically anchored fortress - maintaining the organism in a closed, low-energy, vegetative state that buffers the vulnerable inner layers from unpredictable reality.
This outermost mirrored ENS role is supported by clinical observations that many psychotic patients remain physically robust, showing few if any somatic complaints, as if the body is preserved in a stable metabolic enclave while higher regulatory layers disintegrate.
It is as if, in psychosis, the organism’s waking state is governed not by the ventral vagal social-engagement system but by a deep-sleep–like mode anchored in ENS–DVNS functioning. In this inverted arrangement, the physiological template of deep non-REM sleep-low metabolic variability, energy conservation, reduced sensorimotor engagement - appears to act as the “higher regulator” of conscious experience. The result is a waking life that is internally cohesive from a metabolic standpoint but disconnected from consensual reality, with thought, perception, and affect unfolding under the rules of a dream-like, internally generated world. This could explain why many psychotic patients show striking bodily resilience, stable appetite, and preserved basic autonomic regulation even while higher-order cognitive and social functions are profoundly disorganized.
The UTAF model explains how neuroception of danger forces a choice: either climb back toward human co-regulation, or descend into the mirrored layers of the non-human hierarchy. In both cases, the same nested autonomic “matryoshkas” are engaged - but in opposite contexts. The human path uses them for integration and real safety; the dark path uses them for isolation, predictability, and withdrawal from reality.
If the need for co-regulation is replaced by dependency on non-human, artificial stimuli -substances, audiovisual environments (AVE), intense sensations, screens, fixed cues, or other patterned inputs - the organism still seeks external regulation, but the source is no longer a living, responsive human. Instead, regulation is provided by a non-human, often human-made artifact that delivers predictable stimulation directly into the nervous system, bypassing the organism’s own capacity for flexible self-regulation. In essence, it is still co-regulation, but with an inanimate partner that never attunes, never repairs, and never evolves.
In the dark matryoshka (Fig. 1, lower half, onto-phylogenetic descent), the innermost layer is a mirrored VVNS. In the UTAF framework, each layer of the dark matryoshka carries its own distinct form of addictive functioning. In this inverted mode, VVNS’s social engagement becomes compulsive attachment to substances, artificial audiovisual environments, repetitive cues, fetishized objects, or ritualized/obsessive behaviors - social surrogates that mimic bonding while eroding real relatedness; SNS’s mobilization manifests as chronic hyperarousal and defensive impulsivity with panic, hypervigilance, irritability, aggression, and persecutory/paranoid ideation; DVNS’s restorative shutdown shifts into pathological withdrawal-stupor, catatonia, psychomotor retardation, mutism, dissociation/derealization/depersonalization, analgesic “numbing,” and trance-like absorption, sometimes with screen-induced synesthetic phenomena; and ENS’s metabolic base anchors autonomic and somatic dysregulation – appetite/sleep/circadian disruption, cenesthopathic experiences, somatic delusions, vegetative regression, and global psychotic disorganization. Across layers, hallmark psychotic features emerge - auditory/visual/olfactory/tactile hallucinations; delusions (persecutory, referential, grandiose, nihilistic, somatic); formal thought disorder (loosening of associations, derailment, tangentiality, neologisms, thought blocking); negative symptoms (affective flattening, alogia, avolition, anhedonia, asociality); impaired insight and sensory gating—constituting a state-locked, illusory “safety” that substitutes prediction and control for genuine integration. These patterns are not arbitrary malfunctions but phylogenetically conserved regulatory programs – ancient survival systems designed to take over when higher layers fail. In the dark hierarchy, however, their orientation is inverted: rather than integrating the organism into the relational world, they funnel regulation inward, fostering isolation and immersion into an internally generated, self-referential reality.
The pathological, maladaptive behavior of individuals affected by trauma or addiction is a form of striving for intrauterine state. Once formed and valued as an ultra-safe world, the state of the dark matryoshka becomes intensely desired by the organism as an experience of extreme protection, which gives rise to craving – behavior that seeks familiar stimulation to enable sinking back into that state: substance use, stimulation, compulsion, self-harm.
However, remaining in the state of the dark matryoshka makes the organism increasingly “unborn,” i.e., disconnected from the external world, sensory deprived. Consequently, each subsequent encounter with reality becomes progressively more inadequate, maladaptive to the world, leading to yet another clash with reality, activation of “danger” mode, and closing the vicious cycle of craving for re-immersion into the mystical safe zone.
At its deepest logic, the core of the dark matryoshka is not merely “dysregulation” but the fusion of trauma and addiction into a single survival strategy – a closed-loop system that becomes the entry point to every other mirrored layer of the hierarchy. This trauma–addiction functioning acts like a locked gateway: it keeps the organism anchored in an illusory safety mode, regulating through predictable, non-human surrogates rather than through the inherently unpredictable, affectively rich, and growth-provoking realm of human co-regulation. In developmental terms, it is as if the person remains suspended in a form of prolonged intrauterine existence – shielded from the noise, novelty, and demands of the outside world, but also cut off from its vitality, reciprocity, and possibility.
Resolving this core layer does not simply “remove symptoms”; it is a metamorphic act. When the trauma–addiction loop is dismantled, the nervous system reopens to the full orchestration of the healthy hierarchy. The person is, in a profound sense, born anew – emerging from the fluid, buffered safety of the intrauterine analogue into the textured, uncertain, yet deeply nourishing reality of human social life. This transition marks not just the cessation of dependency but the reclamation of agency, curiosity, and authentic connection, enabling the individual to inhabit the real world as a participant rather than a distant observer.
Conclusion
The Unified Traumatic–Addictive Functioning (UTAF) model reframes trauma, addiction, and psychosis not as disparate disorders, but as different expressions of the same hierarchical regulatory architecture operating under divergent conditions. By mapping both the adaptive “human” matryoshka and its mirrored “dark” counterpart, UTAF reveals how the same autonomic, predictive, and learning systems can either sustain integration and social engagement – or spiral into isolation, compulsive regulation, and regression.
The model clarifies that the outermost layer of the dark hierarchy – the trauma–addiction loop – is the gateway to all deeper regressions. This loop, driven by mirrored ventral-vagal mechanisms, replaces genuine human co-regulation with non-human surrogates: substances, artificial audiovisual environments, compulsive behaviors, and rigid sensory cues. While these provide momentary state relief, they also entrench the nervous system in an illusory safety mode, progressively distancing it from authentic social and sensory integration.
Resolving this outer loop is therefore the essential therapeutic target. It is not merely about abstaining from the addictive object or dampening symptoms – it is about restoring the nervous system’s ability to re-enter the human hierarchy, reclaim flexible access to all autonomic platforms, and dismantle the mirrored survival scaffolding layer by layer. Once this process succeeds, the person is, in a profound sense, born anew – emerging from the buffered predictability of the intrauterine analogue into the open, unpredictable, yet nourishing reality of human connection.
Addiction and trauma are inextricably linked not only in terms of shared symptomatology and manifestation correlations, but because addictive functioning is an aspect of trauma that creates it, sustains it, and complicates it over time. A mode of functioning in which the organism strives for intrauterine state – characterized by sensory deprivation, widely described in psychopathology – is addictive functioning: the organism has become dependent precisely on this state, experienced as ultra-safe and therefore valued as “the winner takes it all.” It is not dependent on the specific substance, stimulation, or compulsion – they are merely the vehicle through which the person can return there. This functioning can form whenever higher regulatory systems have been unable to offer a solution and this reaction becomes generalized (in traumatic events or chronic stress), or when, through a particular substance or stimulation, the organism drops directly into this mode and there experiences the sacred safety.
The only competing model of functioning – totally opposed to intrauterine psychoticity – that can provide an absolute sense of safety while keeping the organism awake and adaptive, and thus fully able to learn and develop, is the experience of self-regulation through VVNS. This is precisely why the capacity for attachment and human relating based on deep connectedness, absence of fear, and cortical alertness in others constitutes the environment for successful treatment and full recovery of people with addiction or trauma.
UTAF’s nested architecture offers both a conceptual and practical roadmap: it identifies the active layer of dysregulation, predicts the forms of maladaptation likely to emerge at each mirrored stage, and provides a rationale for phased interventions. By integrating insights from polyvagal theory, developmental neuroscience, and clinical observation, the model bridges the conceptual gap between trauma therapy, addiction treatment, and severe mental illness – pointing toward a unified, state-first, regulation-driven approach.
In this light, healing is not the erasure of the dark matryoshka, but its re-integration under ventral-vagal leadership. Only then can the individual move fluidly between mobilization, rest, and connection – living not in survival’s shadow, but in the full spectrum of human experience.
References
Alaba-Ekpo, O., Caudwell, K. M., & Flack, M. (2024). Examining the strength of the association between problem gambling and gambling to escape: A systematic review and meta-analysis. International Journal of Mental Health and Addiction. Advance online publication. https://doi.org/10.1007/s11469-024-01354-5
Amani, E. (2023). The association between compulsive buying disorder and childhood trauma. Global Journal of Intellectual & Developmental Disabilities, 12(2), 555833. https://doi.org/10.19080/GJIDD.2023.12.555833
Amstadter, A. B., Lönn, S., Sundquist, J., Sundquist, K., & Kendler, K. S. (2023). Post-traumatic stress disorder and drug use disorder: examination of aetiological models in a Swedish population-based cohort. European Journal of Psychotraumatology, 14(2). https://doi.org/10.1080/20008066.2023.2258312
Austin, M. A., Riniolo, T. C., & Porges, S. W. (2007). Borderline personality disorder and emotion regulation: Insights from the Polyvagal Theory. Brain and cognition, 65(1), 69-76. https://doi.org/10.1016/j.bandc.2006.05.007
Bach, J. F., Postaire, É., & Bernard, A. (2013). L'enfant et les écrans. Académie des sciences (France), Le Pommier.
Back, S. E., Jarnecke, A. M., Norman, S. B., Zaur, A. J., & Hien, D. A. (2024). State of the Science: Treatment of comorbid posttraumatic stress disorder and substance use disorders. Journal of traumatic stress, 37(6), 803–813. https://doi.org/10.1002/jts.23049
Berard, M., Peries, M., Loubersac, J., Picot, M.-C., Bernard, J. Y., Munir, K., & Baghdadli, A., for the ELENA study group. (2022). Screen time and associated risks in children and adolescents with autism spectrum disorders during a discrete COVID-19 lockdown period. Frontiers in Psychiatry, 13, 1026191. https://doi.org/10.3389/fpsyt.2022.1026191
Bölte, S., Girdler, S., & Marschik, P. B. (2019). The contribution of environmental exposure to the etiology of autism spectrum disorder. Cellular and molecular life sciences, 76(7), 1275-1297. https://doi.org/10.1007/s00018-018-2988-4
Brownback, T., & Mason, L. (1999). Neurotherapy in the treatment of dissociation. In Introduction to quantitative EEG and neurofeedback (pp. 145-156). Academic Press. https://doi.org/10.1016/B978-012243790-8/50007-9
Carter CS (2003) Developmental consequences of oxytocin. Physiol Behav 79: 383–397. doi: DOI: 10.1016/s0031-9384(03)00151-3
Carter CS (2007) Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav Brain Res 176:170–186. https://doi.org/10.1016/j.bbr.2006.08.025
Carter CS (2014) Oxytocin pathways and the evolution of human behavior. Annu Rev Psychol 65:17–39. doi: 10.1146/annurev-psych-010213-115110
Carter CS (2017) The oxytocin-vasopressin pathway in the context of love and fear. Front Endocrinol (Lausanne) 8:356. doi: 10.3389/fendo.2017.00356
Carter, C. S., Kenkel, W. M., MacLean, E. L., Wilson, S. R., Perkeybile, A. M., Yee, J. R., ... & Kingsbury, M. A. (2020). Is oxytocin “nature’s medicine”?. Pharmacological reviews, 72(4), 829-861. https://doi.org/10.1124/pr.120.019398
Chonchaiya, W., Nuntnarumit, P., & Pruksananonda, C. (2011). Comparison of television viewing between children with autism spectrum disorder and controls. Acta Paediatrica, 100(7), 1033-1037. https://doi.org/10.1111/j.1651-2227.2011.02166.x
Cloitre, M. (2020). ICD-11 complex post-traumatic stress disorder: simplifying diagnosis in trauma populations. The British Journal of Psychiatry, 216(3), 129–131. doi:10.1192/bjp.2020.43
Cogan, N., Morton, L., Campbell, J., Irvine Fitzpatrick, L., Lamb, D., De Kock, J., … Porges, S. (2025). Neuroception of psychological safety scale (NPSS): validation with a UK based adult community sample. European Journal of Psychotraumatology, 16(1). https://doi.org/10.1080/20008066.2025.2490329
Elkin, N., Ashraf, A. K. M., Kılınçel, O., Kılınçel, Ş., Ranganathan, M., Sakarya, A. K., & Soydan, A. M. (2025). Screens and scars: SEM analysis of the relationship between childhood trauma, emotion regulation, and social media addiction. Frontiers in Psychology, 16, 1502968. https://doi.org/10.3389/fpsyg.2025.1502968
Graap, K., & Freides, D. (1998). Regarding the database for the Peniston alpha-theta EEG biofeedback protocol. Applied psychophysiology and biofeedback, 23(4), 265-272. https://doi.org/10.1023/A:1022265716026
Grubbs, J. B., Chapman, H., & Shepherd, K. A. (2018). Post-traumatic stress and gambling-related cognitions: Analyses in inpatient and online samples. Addictive Behaviors, 89, 128–135. https://doi.org/10.1016/j.addbeh.2018.09.035
Haller, M., & Chassin, L. (2014). Risk pathways among traumatic stress, posttraumatic stress disorder symptoms, and alcohol and drug problems: a test of four hypotheses. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors, 28(3), 841–851. https://doi.org/10.1037/a0035878
Harlé, B., & Desmurget, M. (2012). Effets de l’exposition chronique aux écrans sur le développement cognitif de l’enfant. Archives de pédiatrie, 19(7), 772-776. https://doi.org/10.1016/j.arcped.2012.04.003
Hawn, S. E., Cusack, S. E., & Amstadter, A. B. (2020). A Systematic Review of the Self-Medication Hypothesis in the Context of Posttraumatic Stress Disorder and Comorbid Problematic Alcohol Use. Journal of traumatic stress, 33(5), 699–708. https://doi.org/10.1002/jts.22521
Heffler, K. F., & Oestreicher, L. M. (2015). Causation model of autism: Audiovisual brain specialization in infancy competes with social brain networks. Medical Hypotheses, 91, 114–122. https://doi.org/10.1016/j.mehy.2015.06.019
Heffler, K. F., Frome, L. R., & Gullo, D. F. (2022). Changes in autism symptoms associated with screen exposure: case report of two young children. Psychiatry Research Case Reports, 1(2), 100059. https://doi.org/10.1016/j.psycr.2022.100059
Heffler, K. F., Frome, L. R., Garvin, B., Bungert, L. M., & Bennett, D. S. (2022). Screen time reduction and focus on social engagement in autism spectrum disorder: A pilot study. Pediatrics International, 64(1), e15343. https://doi.org/10.1111/ped.15343
Heffler, K. F., Sienko, D. M., Subedi, K., McCann, K. A., & Bennett, D. S. (2020). Association of early-life social and digital media experiences with development of autism spectrum disorder-like symptoms. JAMA Pediatrics. https://doi.org/10.1001/jamapediatrics.2020.0230
Hildebrand, A., Behrendt, S., & Hoyer, J. (2014). Treatment outcome in substance use disorder patients with and without comorbid posttraumatic stress disorder: A systematic review. Psychotherapy Research, 25(5), 565–582. https://doi.org/10.1080/10503307.2014.923125
Houghton, D. C., Spratt, H. M., Keyser-Marcus, L., et al. (2023). Behavioral and neurocognitive factors distinguishing post-traumatic stress comorbidity in substance use disorders. Translational Psychiatry, 13, 296. https://doi.org/10.1038/s41398-023-02591-3
Hutton, J. S., Dudley, J., DeWitt, T., & Horowitz-Kraus, T. (2022). Associations between digital media use and brain surface structural measures in preschool-aged children. Scientific reports, 12(1), 19095. https://doi.org/10.1038/s41598-022-20922-0
Hutton, J. S., Dudley, J., Horowitz-Kraus, T., DeWitt, T., & Holland, S. K. (2020). Associations between screen-based media use and brain white matter integrity in preschool-aged children. JAMA pediatrics, 174(1), e193869-e193869. doi:10.1001/jamapediatrics.2019.3869
Imperatori, C., Barchielli, B., Corazza, O., Carbone, G. A., Prevete, E., Montaldo, S., … Bersani, F. S. (2023). The Relationship Between Childhood Trauma, Pathological Dissociation, and Behavioral Addictions in Young Adults: Findings from a Cross-Sectional Study. Journal of Trauma & Dissociation, 24(3), 348–361. https://doi.org/10.1080/15299732.2023.2181479
Irner, T. B. (2012). Substance exposure in utero and developmental consequences in adolescence: a systematic review. Child Neuropsychology, 18(6), 521-549. https://doi.org/10.1080/09297049.2011.628309
Janecka, M., Kodesh, A., Levine, S. Z., Lusskin, S. I., Viktorin, A., Rahman, R., ... & Reichenberg, A. (2018). Association of autism spectrum disorder with prenatal exposure to medication affecting neurotransmitter systems. Jama Psychiatry, 75(12), 1217-1224. doi:10.1001/jamapsychiatry.2018.2728
Kabali, H. K., Irigoyen, M. M., Nunez-Davis, R., Budacki, J. G., Mohanty, S. H., Leister, K. P., & Bonner Jr, R. L. (2015). Exposure and use of mobile media devices by young children. Pediatrics, 136(6), 1044-1050. https://doi.org/10.1542/peds.2015-2151
Karatzias, T., Murphy, P., Cloitre, M., Bisson, J., Roberts, N., Shevlin, M., Hyland, P., Maercker, A., Ben-Ezra, M., Coventry, P., Mason-Roberts, S., Bradley, A., & Hutton, P. (2019). Psychological interventions for ICD-11 complex PTSD symptoms: systematic review and meta-analysis. Psychological medicine, 49(11), 1761–1775. https://doi.org/10.1017/S0033291719000436
Kolacz, J., Kovacic, K. K., & Porges, S. W. (2019). Traumatic stress and the autonomic brain‐gut connection in development: Polyvagal theory as an integrative framework for psychosocial and gastrointestinal pathology. Developmental psychobiology, 61(5), 796-809. https://doi.org/10.1002/dev.21852
Kolacz, J., Porges, S.W. (2024). Social Co-regulation of the Autonomic Nervous System Between Infants and Their Caregivers. In: Osofsky, J.D., Fitzgerald, H.E., Keren, M., Puura, K. (eds) WAIMH Handbook of Infant and Early Childhood Mental Health. Springer, Cham. https://doi.org/10.1007/978-3-031-48627-2_11
Kolacz, J., Tabares, J. V., Roath, O. K., Rooney, E., Secor, A., Nix, E. J., Tomlinson, C. A., & Bryan, C. J. (2025). Dynamics of PTSD and autonomic symptoms in a longitudinal U.S. population-based sample.Psychological Trauma: Theory, Research, Practice, and Policy. Advance online publication. https://doi.org/10.1037/tra0001918
Konkolÿ Thege, B., Horwood, L., Slater, L., Tan, M. C., Hodgins, D. C., & Wild, T. C. (2017). Relationship between interpersonal trauma exposure and addictive behaviors: a systematic review. BMC psychiatry, 17(1), 164. https://doi.org/10.1186/s12888-017-1323-1
Koob, G. F. (2013). Negative reinforcement in drug addiction: The darkness within. Current Opinion in Neurobiology, 23(4), 559–563. https://doi.org/10.1016/j.conb.2013.03.011
Koob, G. F., & Volkow, N. D. (2016). Neurobiology of addiction: a neurocircuitry analysis. The lancet. Psychiatry, 3(8), 760–773. https://doi.org/10.1016/S2215-0366(16)00104-8
Kosten, T. R., & George, T. P. (2002). The neurobiology of opioid dependence: implications for treatment. Science & practice perspectives, 1(1), 13–20. https://doi.org/10.1151/spp021113
Kosten, T. R., & George, T. P. (2002). The neurobiology of opioid dependence: implications for treatment. Science & practice perspectives, 1(1), 13–20. https://doi.org/10.1151/spp021113
Kwako, L. E., & Koob, G. F. (2017). Neuroclinical framework for the role of stress in addiction. Chronic Stress, 1, 2470547017698140. https://doi.org/10.1177/2470547017698140
Levin, Y., Lev Bar-Or, R., Forer, R., Vaserman, M., Kor, A., & Lev-Ran, S. (2021). The association between type of trauma, level of exposure and addiction. Addictive Behaviors, 118, 106889. https://doi.org/10.1016/j.addbeh.2021.106889
Levine, P. A. (1997). Waking the tiger: Healing trauma: The innate capacity to transform overwhelming experiences. North Atlantic Books.
Levine, P. A. (2010). In an unspoken voice: How the body releases trauma and restores goodness. North Atlantic Books.
Levine, P. A., & Kline, M. (2006). Trauma through a child's eyes: Awakening the ordinary miracle of healing. North Atlantic Books.
Levine, P. A., Blakeslee, A., & Sylvae, J. (2018). Reintegrating fragmentation of the primitive self: Discussion of “somatic experiencing”. Psychoanalytic Dialogues, 28(5), 620-628. https://doi.org/10.1080/10481885.2018.1506216
Lima, K. H. M., Gomes, J. S., & Tucci, A. M. (2022). Electroencephalographic neurofeedback as a tool for reducing harm and risk associated with alcohol use disorder: a critical review. Drug and Alcohol Review, 41(3), 594-602. https://doi.org/10.1111/dar.13387
Liu, Q., Ouyang, L., Fan, L., Wang, L., Zhang, Y., & Liang, Y. (2024). Association between childhood trauma and Internet gaming disorder: A moderated mediation analysis with depression as a mediator and psychological resilience as a moderator. BMC Psychiatry, 24, 412. https://doi.org/10.1186/s12888-024-05863-4
Luciano, M. T., Acuff, S. F., Olin, C. C., Lewin, R. K., Strickland, J. C., McDevitt-Murphy, M. E., & Murphy, J. G. (2022). Posttraumatic stress disorder, drinking to cope, and harmful alcohol use: A multivariate meta-analysis of the self-medication hypothesis. Journal of psychopathology and clinical science, 131(5), 447–456. https://doi.org/10.1037/abn0000764
Manolova V.R. and Vezenkov S.R. (2025) Are Screen-Induced Synesthesia and Screen-induced Cue-Dependent Behavior Contributing Factors in the Misdiagnosis of ASD Among Children with Screen Addiction? Nootism 1(3), 11-16. https://doi.org/10.64441/nootism.1.3.2 (2)
Manolova V.R. and Vezenkov S.R. (2025) Screen Trauma – Specifics of the Disorder and Therapy in Adults and Children. Nootism 1(1), 37-51. https://doi.org/10.64441/nootism.2NCSC.3 (1)
Manolova, V.R., Pashina, I.H., Mateev M.I and Vezenkov, S.R. (2025) Munchausen Syndrome by Proxy and Other Forms of Parental Abuse in Children with Screen Addiction and a Diagnosis of Autism (ASD) and/or ADHD. Nootism 1(2), 11-30. https://doi.org/10.64441/nootism.1.2.2
Marcelli, D., Bossière, M.-C. et Ducanda, A.-L. (2018). Plaidoyer pour un nouveau syndrome « Exposition précoce et excessive aux écrans » (epee) Enfances & Psy, 79(3), 142-160. https://doi.org/10.3917/ep.079.0142
Marcelli, D., Bossière, M.-C. et Ducanda, A.-L. (2020). L’exposition précoce et excessive aux écrans (EPEE) : un nouveau syndrome. Devenir, . 32(2), 119-137. https://doi.org/10.3917/dev.202.0119
María-Ríos, C. E., & Morrow, J. D. (2020). Mechanisms of shared vulnerability to post-traumatic stress disorder and substance use disorders. Frontiers in behavioral neuroscience, 14, https://doi.org/10.3389/fnbeh.2020.00006
Monson, E. Villotti, P., & Hack, B. (2023). Trauma and gambling: A scoping review of qualitative research. Critical Gambling Studies, 4(1), 12-26. https://doi.org/10.29173/cgs113
Neophytou, E., Manwell, L. A., & Eikelboom, R. (2021). Effects of excessive screen time on neurodevelopment, learning, memory, mental health, and neurodegeneration: A scoping review. International Journal of Mental Health and Addiction, 19(3), 724-744. https://doi.org/10.1007/s11469-019-00182-2
Nigam, T., & Schwiedrzik, C. M. (2024). Predictions enable top-down pattern separation in the macaque face-processing hierarchy. Nature Communications, 15(1), 7196. https://doi.org/10.1038/s41467-024-51543-y
Pagani, L. S., Fitzpatrick, C., Barnett, T. A., & Dubow, E. (2010). Prospective associations between early childhood television exposure and academic, psychosocial, and physical well-being by middle childhood. Archives of Pediatrics & Adolescent Medicine, 164(5), 425–431. https://doi.org/10.1001/archpediatrics.2010.50
Patel, H., Easterbrook, B., Ralston, F. A., Shariff, D., Lester, H., Landaverde, D., Lau, E., Davis, I. S., Aks, I. R., Brown, S. A., Tapert, S. F., & Pelham, W. E. III. (2025). Increased prevalence of childhood complex trauma in comorbid posttraumatic stress disorder and substance use disorders compared to either disorder alone: A systematic review. Early Intervention in Psychiatry. Advance online publication. https://doi.org/10.1111/eip.70051
Peniston, E. G., & Kulkosky, P. J. (1989). α‐θ Brainwave Training and β‐Endorphin Levels in Alcoholics. Alcoholism: Clinical and experimental research, 13(2), 271-279. https://doi.org/10.1111/j.1530-0277.1989.tb00325.x
Peniston, E. G., & Kulkosky, P. J. (1999). Neurofeedback in the treatment of addictive disorders. In Introduction to quantitative EEG and neurofeedback (pp. 157-179). Academic Press. https://doi.org/10.1016/B978-012243790-8/50008-0
Petkova, S. P., Manolova, V. R., and Vezenkov, S. R. (2025). Restoring attachment in children with early screen addiction. Nootism 1(1), 74-78. https://doi.org/10.64441/nootism.2NCSC.7
Petrova, S.N., Manolova V.R. and Vezenkov, S.R. (2025) Reintroducing Screens: Severe Regression and Symptom Aggravation in Children with ASD/Screen Addiction. Nootism 1(1), 59-65, https://doi.org/10.64441/nootism.2NCSC.5
Porges, S. W. (1995). Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology, 32(4), 301-318. https://doi.org/10.1111/j.1469-8986.1995.tb01213.x
Porges, S. W. (2001). The polyvagal theory: phylogenetic substrates of a social nervous system. International journal of psychophysiology, 42(2), 123-146. https://doi.org/10.1016/S0167-8760(01)00162-3
Porges, S. W. (2003). The polyvagal theory: Phylogenetic contributions to social behavior. Physiology & behavior, 79(3), 503-513. https://doi.org/10.1016/S0031-9384(03)00156-2
Porges, S. W. (2007). The polyvagal perspective. Biological psychology, 74(2), 116-143. https://doi.org/10.1016/j.biopsycho.2006.06.009
Porges, S. W. (2009). The polyvagal theory: New insights into adaptive reactions of the autonomic nervous system. Cleveland Clinic journal of medicine, 76(Suppl 2), S86. doi: 10.3949/ccjm.76.s2.17
Porges, S. W. (2011). The polyvagal theory: Neurophysiological foundations of emotions, attachment, communication, and self-regulation (Norton series on interpersonal neurobiology). WW Norton & Company.
Porges, S. W. (2021). Polyvagal Theory: A biobehavioral journey to sociality. Comprehensive Psychoneuroendocrinology, 7, 100069.
Porges, S. W. (2022). Polyvagal theory: A science of safety. Frontiers in integrative neuroscience, 16, 871227. https://doi.org/10.3389/fnint.2022.871227
Porges, S. W. (2023). The vagal paradox: A polyvagal solution. Comprehensive psychoneuroendocrinology, 16, 100200. https://doi.org/10.1016/j.cpnec.2023.100200
Porges, S. W. (2024). Polyvagal theory: The neuroscience of safety in trauma-informed practice. The Handbook of Trauma-Transformative Practice: Emerging Therapeutic Frameworks for Supporting Individuals, Families or Communities Impacted by Abuse and Violence, 51. https://books.google.de/books?hl=bg&lr=&id=JmPHEAAAQBAJ&oi=fnd&pg=PA51&dq=porges+polyvagal+theory&ots=T8MPg4abAx&sig=_VRfIuDjwkWkgNcmedxDJgxSmYY&redir_esc=y#v=onepage&q=porges polyvagal theory&f=false
Porges, S. W. (2025). Deconstructing Autism Through the Lens of the Polyvagal Theory: A foreword to Understanding Autism (D. Papaneophytou & S. Das, Eds., pp. xxiii–xxx). In Understanding Autism. https://doi.org/10.1016/B978-0-443-27366-7.09998-3
Porges, S. W., & Lewis, G. F. (2010). The polyvagal hypothesis: common mechanisms mediating autonomic regulation, vocalizations and listening. In Handbook of behavioral neuroscience (Vol. 19, pp. 255-264). Elsevier https://doi.org/10.1016/B978-0-12-374593-4.00025-5
Porges, S. W., Doussard-Roosevelt, J. A., Stifter, C. A., McClenny, B. D., & Riniolo, T. C. (1999). Sleep state and vagal regulation of heart period patterns in the human newborn: an extension of the polyvagal theory. Psychophysiology, 36(1), 14-21. https://doi.org/10.1017/S004857729997035X
Porges, S.W. (2007) A phylogenetic journey through the vague and ambiguous Xth cranial nerve: A commentary on contemporary heart rate variability research. Biological Psychology 74 (2007) 301–307 https://doi.org/10.1016/j.biopsycho.2006.08.007
Porges, S.W. (2025) Polyvagal Theory: Current Status, Clinical Applications, and Future Directions. Clinical Neuropsychiatry 22(3), 169-184 DOI: 10.36131/cnfioritieditore20250301
Poulain, T., Vogel, M., Neef, M., Abicht, F., Hilbert, A., Genuneit, J., ... & Kiess, W. (2018). Reciprocal associations between electronic media use and behavioral difficulties in preschoolers. International journal of environmental research and public health, 15(4), 814. https://doi.org/10.3390/ijerph15040814
Renaud, F., Jakubiec, L., Swendsen, J., & Fatseas, M. (2021). The impact of co-occurring post-traumatic stress disorder and substance use disorders on craving: A systematic review of the literature. Frontiers in Psychiatry, 12, 786664. https://doi.org/10.3389/fpsyt.2021.786664
Renaud, F., Jakubiec, L., Swendsen, J., & Fatseas, M. (2021). The impact of co-occurring post-traumatic stress disorder and substance use disorders on craving: A systematic review of the literature. Frontiers in Psychiatry, 12, 786664. https://doi.org/10.3389/fpsyt.2021.786664
Richter D, Kietzmann TC, de Lange FP (2024) High-level visual prediction errors in early visual cortex. PLoS Biol 22(11): e3002829. https://doi.org/10.1371/journal.pbio.3002829Russo, G. M., & Novian, D. A. (2014). A research analysis of neurofeedback protocols for PTSD and alcoholism. NeuroRegulation, 1(2), 183-183. https://doi.org/10.15540/nr.1.2.183
Russo, G. M., Smith, S., & Sperandio, K. R. (2023). A meta‐analysis of neurofeedback for treating substance use disorders. Journal of Counseling & Development, 101(2), 143-156. https://doi.org/10.1002/jcad.12466
Sandtorv, L. B., Fevang, S. K. E., Nilsen, S. A., Bøe, T., Gjestad, R., Haugland, S., & Elgen, I. B. (2018). Symptoms associated with attention deficit/hyperactivity disorder and autism spectrum disorders in school-aged children prenatally exposed to substances. Substance Abuse: Research and Treatment, 12, 1178221818765773. https://doi.org/10.1177/1178221818765773
Sheng, X., Yang, M., Ge, M., Zhang, L., Huang, C., Cui, S., Yuan, Q., Ye, M., Zhou, R., Cao, P., Peng, R., Zhang, K., & Zhou, X. (2022). The relationship between Internet addiction and childhood trauma in adolescents: The mediating role of social support. Frontiers in Psychology, 13, 996086. https://doi.org/10.3389/fpsyg.2022.996086
Shi, L., Wang, Y., Yu, H., Wilson, A., Cook, S., Duan, Z., Peng, K., Hu, Z., Ou, J., Duan, S., Yang, Y., Ge, J., Wang, H., Chen, L., Zhao, K., & Chen, R. (2020). The relationship between childhood trauma and Internet gaming disorder among college students: A structural equation model. Journal of behavioral addictions, 9(1), 175–180. https://doi.org/10.1556/2006.2020.00002
Simonato, I., Janosz, M., Archambault, I., & Pagani, L. S. (2018). Prospective associations between toddler televiewing and subsequent lifestyle habits in adolescence. Preventive medicine, 110, 24-30. https://doi.org/10.1016/j.ypmed.2018.02.008
Stefanova M.Ts., Manolova, V.R. and Vezenkov S.R. (2025) ADHD and Screen Addiction in Children Aged 3-9: Staged Recovery and Neurophysiological Markers. Nootism 1(1), 66-73. https://doi.org/10.64441/nootism.2NCSC.6
Trocki, K. F. (2006). Is there an Anti-Neurofeedback Conspiracy?. Journal of Addictions Nursing, 17(4), 199-202. DOI: 10.1080/10884600600995176
Vezenkov, S.R. and Manolova V.R. (2025) Neurobiology of Autism/Early Screen Addiction Recovery. Nootism 1(1), 19-36. https://doi.org/10.64441/nootism.2NCSC.2 (1)
Vezenkov, S.R. and Manolova, V.R. (2024) Rethinking Autism: The Screen Addiction Paradigm. BFE 22nd Meeting, 8-13 April 2024, Ljubljana, Slovenia, doi: 10.13140/RG.2.2.27970.80328
Vezenkov, S.R. and Manolova, V.R. (2025) Gaming and Gambling Addiction in Fathers as a Risk Factor for Early Screen Addiction in Children. Nootism 1(2), 31-40. https://doi.org/10.64441/nootism.1.2.3 (2)
Vezenkov, S.R. and Manolova, V.R. (2025) Screen Addiction – Biomarkers, Developmental Damage and Recovery. Nootism 1(1), 6-18. https://doi.org/10.64441/nootism.2NCSC.1 (4)
Vezenkov, S.R. and Manolova, V.R. (2025) Screen-Induced Pathological Eye-Covering Reflex in Children with Early Screen Addiction. Nootism 1(3), 5-10. https://doi.org/10.64441/nootism.1.3.1 (3)
Vezenkov, S.R. and Manolova, V.R. (2025) Screen-Induced Pathological Vestibular Reflex: A Specific Marker of Early Screen Addiction. Nootism 1(2), 5-10. https://doi.org/10.64441/nootism.1.2.1 (5)
Wang, X., Sun, F., Geng, F., Liu, H., Zhang, J., Liang, L., & Huang, X. (2025). The relationship between childhood trauma and internet addiction in adolescents with depression: The mediating role of insomnia and alexithymia. BMC Psychiatry, 25, 298. https://doi.org/10.1186/s12888-025-06739-x
White, N. E. (2008). The transformational power of the Peniston protocol: A therapist's experiences. Journal of Neurotherapy, 12(4), 261-265. https://doi.org/10.1080/10874200802502383
Wise, R., & Koob, G. (2014). The development and maintenance of drug addiction. Neuropsychopharmacology, 39(2), 254–262. https://doi.org/10.1038/npp.2013.261
Wu, X., Tao, S., Rutayisire, E., Chen, Y., Huang, K., & Tao, F. (2017). The relationship between screen time, nighttime sleep duration, and behavioural problems in preschool children in China. European child & adolescent psychiatry, 26(5), 541-548. https://doi.org/10.1007/s00787-016-0912-8
Yao, Q., Gao, Y., Liu, C., et al. (2025). The impact of childhood trauma on short video addiction: Psychological and morphological correlates. Scientific Reports, 15, 18999. https://doi.org/10.1038/s41598-025-04020-5
Zamfir, M. T. (2018). The consumption of virtual environment more than 4 hours/day, in the children between 0-3 years old, can cause a syndrome similar with the autism spectrum disorder. Journal of Romanian literary studies, (13), 953-968. https://www.ceeol.com/search/article-detail?id=742946