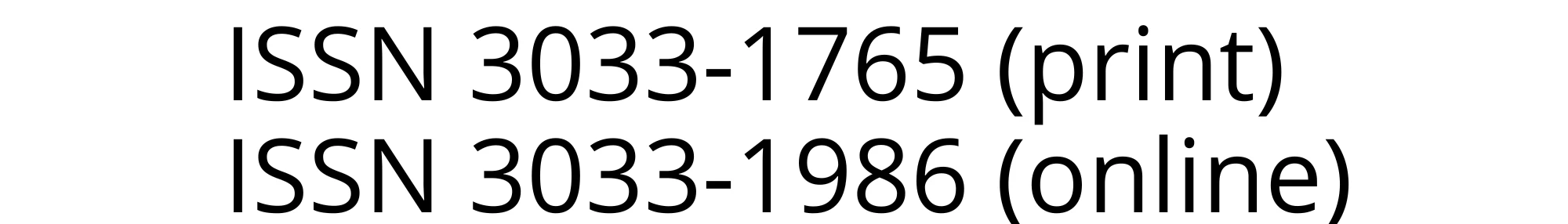Stoyan R. Vezenkov and Violeta R. Manolova
Center for applied neuroscience Vezenkov, BG-1582 Sofia, e-mail: info@vezenkov.com
For citation: Vezenkov, S.R. and Manolova, V.R. (2025) A Three‑Stage Autonomic Biofeedback Program Improves IBS Symptoms and Quality of Life. Nootism 1(5), 4-20, https://doi.org/10.64441/nootism.1.5.1
Abstract
Background. Irritable bowel syndrome (IBS) is a prevalent disorder of gut–brain interaction with substantial quality‑of‑life (QoL) burden and heterogeneous mechanisms, motivating individualized, mechanism‑informed care.
Methods. We conducted a single‑arm, prospective pre–post study of 120 enrolled adults with IBS offered a 12‑week, three‑stage, therapist‑delivered program integrating psychoeducation; autonomic self‑regulation with heart‑rate variability (HRV), skin conductance level (SCL), and peripheral temperature biofeedback; targeted interoceptive provocation paired with real‑time autonomic stabilization; and psychosocial trigger work with relapse‑prevention planning. Pelvic‑floor/anorectal sEMG biofeedback was not included. The primary outcome was IBS‑SSS total (0–500); secondary outcomes were IBS‑QoL total (sum of 34 items; each 1–5 transformed to 0-100) and eight QoL subscales. Analyses used paired tests with effect sizes and FDR correction for subscales; exploratory correlations linked ΔIBS‑SSS to ΔIBS‑QoL.
Results. Of 120 enrolled, 84 (70.0%) completed all 12 sessions and both assessments (drop‑out 30.0%). In the complete‑case cohort (n=84), IBS‑SSS decreased from 307.7 ± 100.2 to 137.0 ± 83.1 (Δ=−170.7; 95% CI −186.7 to −154.7; d<sub>z</sub>=2.32; p≈2.6×10⁻³⁵; Wilcoxon p≈1.71×10⁻¹⁵). The IBS‑QoL mean paired increase was +45.17 points (95% CI +41.89 to +48.46; d<sub>z</sub> = 2.98; t(83) = 27.35, p = 2.80×10⁻⁴³; Wilcoxon p = 1.71×10⁻¹⁵). All eight domains improved substantially and significantly after the program (q ≪ 0.001 across all subscales). The largest average gains were in Health Worry and Food Avoidance, followed by Social Reaction and Sexual function; Body Image improved the least. Symptom improvement correlated moderately with QoL gains (Pearson r≈−0.342). There was no evidence of sex moderation (ΔSeverity×Sex: b = +0.0422, SE = 0.0584, t = 0.72, p = 0.472), indicating similar severity–QoL coupling in women and men. The ΔSeverity×Age interaction was small and non-significant (b = +0.0030 per year, SE = 0.0018, t = 1.69, p = 0.0943); the point estimate corresponds to a very slight attenuation of the slope with older age (about 3 QoL points less per 100-point IBS-SSS decrease for someone 10 years above the sample mean), but this did not reach conventional thresholds.
Interpretation. A structured, autonomic‑anchored complex therapy was feasible and associated with large, clinically meaningful improvements in IBS symptoms and QoL across multiple domains – particularly health worry, food avoidance, and social reaction –that are not uniformly addressed by diet or drugs alone. Causal inference is limited by the uncontrolled design and 30% attrition; a randomized controlled trial is warranted to test the incremental value of autonomic biofeedback within integrated IBS care and to evaluate durability and mechanistic biomarkers alongside patient‑reported outcomes.
Keywords: Irritable Bowel Syndrome; Disorders of Gut–Brain Interaction; Heart Rate Variability (HRV) Biofeedback; Electrodermal Activity (Skin Conductance); Peripheral Temperature Biofeedback; Autonomic Nervous System Regulation; Interoceptive Exposure; Resonance Frequency; Health‑Related Quality of Life (IBS‑QoL); IBS Symptom Severity Scale (IBS‑SSS).
Introduction
Diagnostic criteria and epidemiology
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder that affects on average about one‑fifth of the population (Canavan et al., 2014). Its prevalence varies widely across countries and regions – from 5% in Sri Lanka and Belgium to 30% in Pakistan and Palestine, with a global average around 15% (Oka et al., 2020; Li et al., 2024)
Clinical symptoms of IBS include abdominal pain or discomfort, stool irregularities and bloating, as well as other somatic, visceral (pain syndromes, overactive bladder and migraine etc.) and psychiatric comorbidities (depression, anxiety, somatization, neuroticism etc.). Defined by the Rome IV criteria, it can be classified as constipation-predominant (IBS-C), diarrhea-predominant (IBS-D), mixed stool pattern (IBS-M), and amorphous type (IBS-U) (Drossman, 2016; Enck et al., 2016). Studies have revealed that IBS is more common in females than in males in the Western world at a ratio of 1.67. (Meleine et al., 2014; Lovell et al., 2012) This may be related to female estrogen, which promotes the improvement of intestinal sensitivity or immune dysfunction. (Meleine et al., 2014; Lovell et al., 2012; Payne, 2004)
Beyond gastrointestinal symptoms, IBS is associated with impaired health‑related quality of life (HRQoL), reduced work productivity, and frequent psychological comorbidity. These features, together with heterogeneous symptom phenotypes, create a compelling need for individualized and mechanism‑informed care. (Lacy et al., 2021)
In addition to people who have been diagnosed with IBS, there is a huge number of undiagnosed individuals who perceive their symptoms as part of their everyday life. These people do not consult physicians about their suffering. The latter is especially true for IBS‑C. In this way, the prevalence of the disorder is far higher than that cited in the scientific literature. Many people who suffer from IBS are dissatisfied with the interventions delivered to them by health‑care providers and feel either misunderstood or treated as hypochondriacs or as mentally ill. The view is widespread in society and among health professionals that IBS is unimportant because it is not life‑threatening. Moreover, the consequences of IBS are often neglected – significant deterioration in quality of life, social isolation, leaving work, worsening relationships, and more. Most people suffering from IBS symptoms worry that they are seriously ill with some disease or will become seriously ill over time, which closes a vicious circle and the symptomatology worsens over the years if not treated successfully. The quality of life is so impaired that it is comparable with other chronic diseases such as diabetes and hepatitis. (Enck et al., 2016) And the annual treatment costs in the USA alone are estimated at 1 billion USD (Ford et al., 2017)
Etiology of IBS
Although the underlying pathogenesis is far from understood, aetiological factors include increased epithelial hyperpermeability, dysbiosis, inflammation, visceral hypersensitivity, epigenetics, psychosocial factors and genetics, and altered brain–gut interactions. (Enck et al., 2016) This network of peripheral and central mechanisms helps explain clinical heterogeneity and variable treatment response, and it motivates integrated therapeutic strategies that address both bottom‑up and top‑down drivers of symptoms.
What is essential for the disease is that it is not associated with structural or biochemical abnormalities that can be established with standard medical tests. The heterogeneity of the factors that lead to the disease is so great that many studies strongly contradict each other and no biomarkers have been derived that can be categorically applied to characterize the main subtypes. The overlap of symptoms among the subtypes and with other gastrointestinal diseases has been studied in detail – functional dyspepsia, heartburn, gastroesophageal reflux disease and nausea disorders of the upper GIS, and diarrhoea, incontinence, pelvic floor dyssynergia and constipation of the lower GIS. (Ford et al., 2013)
IBS has been included in the term ‘somatic symptom disorder’ in the Diagnostic and Statistical Manual of Mental Disorders 5th Edition (DSM‑5) and in psychiatric or psychosomatic clinical management. Patients with IBS who were treated by psychiatrists frequently did not receive adequate attention with respect to their gastrointestinal symptoms before the release of DSM‑5. (Enck et al., 2016)
Risk factors include age over 50 years, female sex, chronic stress, violence and traumatic experiences, anxiety, depression, somatization and neuroticism, addictions, change of workplace, bachelor syndrome, and toxic stress (family members with addiction, mental illness, aggressive behavior).
Diagnosis and therapy of IBS
A detailed guideline for diagnosis and therapy is provided in (Lacy et al., 2021). The main recommendations are first to perform differential diagnosis for celiac disease and inflammatory bowel disease. Unnecessary interventions should be avoided, such as testing for enteric pathogens, testing for food allergies and food sensitivities, colonoscopy in persons under 45 years of age; and a positive diagnostic strategy, rather than an exclusionary one as before Rome IV, is recommended in order to avoid unnecessary medical costs without result or utility for subsequent therapy. It is recommended to perform an anorectal physiological test in refractory constipation that is not affected by standard medical therapy and, if the test is positive, to prescribe EMG biofeedback therapy, which is effective and specific, the best compared with any other therapy.
The elimination of dietary fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) has quickly gained popularity as a treatment for patients with IBS. The low‑FODMAP diet seems safe without serious adverse events (AEs), although long‑term over‑restriction of FODMAPs may lead to micronutrient deficiencies. Soluble, viscous, poorly fermentable fiber, but not insoluble, may provide benefits in IBS.
Contemporary guidance supports a stepped, multimodal approach combining dietary, pharmacologic, and brain–gut behavioral therapies. The strongest diet evidence base is for the low‑FODMAP diet, which consistently outperforms habitual or general dietary advice for global symptoms and bloating in randomized trials and network meta‑analyses. A recent, comprehensive network meta‑analysis (28 trials; 2,338 participants) confirmed that low‑FODMAP has the most consistent evidence across endpoints, while other promising strategies (e.g., starch‑/sucrose‑reduced diets, selected “simple” FODMAP exclusions, Mediterranean‑style patterns) merit further comparative trials. (Cuffe et al., 2025)
Antispasmodics remain one of the most frequently used treatments for IBS. They relax intestinal smooth muscle, thereby reducing GI motility – direct smooth‑muscle relaxants, calcium antagonists, scopolamine derivatives, and combination agents. Side effects are common, particularly in the elderly, and the recommendation is against their use in IBS patients. The exact opposite applies to peppermint, which is recommended without strong evidence of efficacy but with indications for influence on symptomatology.
Pharmacologic choices are tailored to the predominant bowel pattern and pain severity. For IBS‑C, secretagogues (linaclotide, plecanatide, lubiprostone) improve global symptoms and bowel function versus placebo in indirect comparisons; for IBS‑D, 5‑HT3, antagonists (alosetron, ramosetron where available) rank among the most effective for composite endpoints, with careful attention to safety and regulatory constraints. Peppermint oil has antispasmodic properties and shows short‑term benefits for pain and global symptoms in meta‑analyses, albeit with low‑certainty evidence; antispasmodics and low‑dose tricyclic antidepressants also have supportive evidence for pain/global symptoms. (Black et al., 2018)
The use of probiotics and laxatives is not recommended in IBS due to the lack of proven positive effect. A good effect has been demonstrated with chloride‑channel activators (e.g., lubiprostone) on the apical membranes of intestinal epithelial cells, but only in IBS‑C. Other drugs for this subtype are guanylate cyclase activators (e.g., plecanatide and linaclotide), called secretagogues. They suggest that the 5‑HT4 agonist tegaserod be used to treat IBS‑C symptoms in women younger than 65 years with ≤1 cardiovascular (CV) risk factors who have not adequately responded to secretagogues. Stimulation of the serotonin type‑4 receptor (5‑HT4) initiates the peristaltic reflex and accelerates GI transit. It is contraindicated in patients with more than one CV risk factor.
They recommend the use of rifaximin, a nonabsorbed antibiotic, to treat global IBS‑D symptoms. Rifaximin treatment is based on the hypothesis that a portion of patients with IBS‑D have an abnormal microbiome. The use of this drug is supported by multiple clinical trials. Serotonin (5‑hydroxytryptamine; 5‑HT) plays an important role in modulating visceral sensation and motility. Patients with IBS‑D have increased plasma levels of serotonin. Alosetron is a 5‑HT3 antagonist, and as such, the primary mechanism of action in the treatment of IBS‑D is the slowing of intestinal transit in women with severe symptoms who have failed conventional therapy.
Antidepressants, such as tricyclic antidepressants (TCAs) or selective serotonin reuptake inhibitors (SSRIs), are recommended by existing guidelines for the treatment of pain in patients who are refractory to antispasmodics and dietary alterations. Adverse effects such as constipation, dry mouth, drowsiness, and fatigue are reported with TCAs. TCAs may be particularly effective for treating pain in patients with IBS‑D, but are less suitable for patients who have IBS‑C. (Enck et al., 2016)
Advances in our understanding of the brain‑gut‑microbiome axis, and the growth of the disciplines of cognitive neurosciences and behavioral intervention science have shown that psychotherapies effective in the treatment of depression, anxiety, and chronic pain can be adapted to manage core symptoms of IBS, including abdominal pain, altered bowel habits, and IBS‑specific health‑related quality of life. Gut‑directed psychotherapies (GDPs), which as a class include cognitive‑behavior therapy (CBT)‑GI, gut‑directed hypnotherapy (GDH), psychodynamic (interpersonal) therapy, mindfulness, and mindfulness‑based stress reduction, improve IBS symptom severity by targeting the cognitive and affective factors known to drive symptom experience. GDPs are IBS‑subtype agnostic and can address the large group of patients with IBS‑M or IBS‑U for whom fewer pharmacological treatments are available. The National Institute of Health and Care Excellence (NICE) guidelines advise that patients whose symptoms do not respond to pharmacological treatments after 12 months and who develop a continuing symptom profile (refractory IBS) should be considered for referral to psychotherapy. This extensive overview of approved therapeutic practices also contains a bibliography of 270 sources. (Lacy et al., 2021)
But whatever data are presented, the general opinion of specialists is summarized in the following conclusion:
Although a substantial proportion of patients will experience spontaneous remission over time, there is currently no treatment that cures IBS; relief of symptoms is the most that can be achieved. (Enck et al., 2016)
Very often medications prescribed by gastroenterologists around the world do not give satisfactory results, despite the huge achievements of pharmacology and well‑controlled clinical studies with randomized control groups and double‑blind experiments. (Palsson et al., 2013) The positive effects in each patient are very different and often decrease over time, while side effects increase and diversify.
Brain–gut behavioral treatments (e.g., cognitive behavioral therapy [CBT] and gut‑directed hypnotherapy [GDH]) are effective and durable for many patients. A large network meta‑analysis concluded that several psychological therapies outperform control conditions, with CBT‑based interventions and GDH supported by the most robust—and often long‑term – evidence; more recent analyses continue to affirm benefits for pain and global outcomes, including digital delivery formats that can broaden access. (Radu et al., 2018)
Mindfulness-Based Stress Reduction (MBSR) has been investigated as a treatment for Irritable Bowel Syndrome (IBS), a functional gastrointestinal disorder heavily influenced by stress. A randomized wait-list controlled trial by Zernicke et al. (2013) examined the impact of an 8-week MBSR program on IBS symptoms and self-reported stress. The study found that patients who underwent the mindfulness program experienced a clinically meaningful reduction in the severity of their IBS symptoms and symptoms of stress immediately following the intervention. These improvements were sustained at a 6-month follow-up, providing preliminary evidence for the feasibility and efficacy of mindfulness-based interventions for managing IBS.
In practice psychotherapies and hypnotherapy also do not give satisfactory results in most patients, because the functional reflex activity of the intestines is difficult to retrain once it has formed and generalized to various environmental stimuli and triggers. The style of coping with stressors can be changed, for example by overcoming the catastrophic and pessimistic perception of oneself and life as a whole, but this is usually a consequence of the symptomatology and not a cause. (Vezenkov, 2016)
Biofeedback therapy for IBS
Two main biofeedback approaches have been described in the therapy of IBS. One is EMG biofeedback for the treatment of dyssynergic defecation with and without IBS, with very high effectiveness and level‑5 efficacy, i.e., the best therapy compared with all other interventions (Koutsomanis et al., 1995; Heymen et al., 2003; Ryan et al., 2004; Chiaroni et l., 2006, 2008; Rao et al., 2007; Ahadi et al., 2014; Cadeddu et al., 2015; Patcharatrakul et al., 2011, 2018; Goldenberg et al., 2019; Hite et al., 2020).
The other is HRV biofeedback, brief interventions of 6 sessions aimed at restoring vagal tone and autonomic balance, which have shown higher effectiveness than hypnotherapy and physical activity in various studies (Bassotti et al., 1994, 1997; Gevirtz, 1999; Lhrer et al., 2000; Del Poso et al., 2002; Sowder et al., 2010; Dobbin et al., 2013; Stern et al., 2014; Vezenkov, 2015; 2016).
Why consider biofeedback within “complex therapy”?
Biofeedback refers to instrument‑guided training that helps patients perceive and modulate physiological processes. In IBS and related DGBI, several mechanistically distinct biofeedback modalities can be deployed, each targeting a specific dysfunction:
-
Anorectal/pelvic floor biofeedback for defecatory disorders (DD). Among constipated patients with pelvic floor dyssynergia—a subgroup that overlaps with IBS‑C—anorectal biofeedback is the treatment of choice, supported by randomized trials (including long‑term follow‑up) and guideline recommendations; effects include improved bowel symptoms and manometric normalization. Although biofeedback treats the defecatory mechanism rather than IBS per se, it is indispensable when DD coexists with IBS. (Rao et al., 2010)
-
Thoracoabdominal wall motion–guided biofeedback for visible abdominal distension. Abdominophrenic dyssynergia (inappropriate diaphragmatic contraction with abdominal wall relaxation) is increasingly recognized in IBS. A 2024 randomized, placebo‑controlled trial showed that real‑time thoracoabdominal motion biofeedback significantly reduced visible distension by training diaphragmatic control—a targeted, low‑risk intervention relevant to a highly bothersome symptom domain. (Barba et al., 2024)
-
Biofeedback‑assisted stress regulation (e.g., respiratory/HRV biofeedback). Pilot randomized trials indicate that brief programs combining relaxation breathing, HRV‑guided feedback, and home practice can reduce perceived stress, depressive symptoms, and IBS symptomatology – consistent with the central role of autonomic regulation in gut–brain disorders – though larger, controlled studies are still needed. (Lehrer & Gevirtz, 2014; Exarchopoulou et al., 2024; Vezenkov, 2015; 2016)
-
EEG neurofeedback (experimental). Early, small RCTs suggest potential benefit on gastrointestinal symptoms and mood indices; however, protocols and targets vary and the evidence remains preliminary compared with other modalities. (Ebrahimi et al., 2023)
Notably, a 2019 Cochrane review concluded that the overall evidence for general “biofeedback for IBS” was insufficient due to small, heterogeneous studies. The field has advanced by matching modality to mechanism (e.g., dyssynergic defecation, abdominophrenic dyssynergia, stress‑autonomic dysregulation), a precision that strengthens the clinical rationale for embedding targeted biofeedback within broader IBS care. (Goldenberg et al., 2019)
Evidence Base Guiding the Complex Therapy Design
Irritable bowel syndrome is heterogeneous in mechanisms and presentation, so our program was built on an evidence gradient that prioritizes modalities with the strongest and most durable benefits while allowing targeted add-ons for specific pathophysiology. At the highest tier, we anchored the intervention in approaches supported by multiple randomized trials and network meta-analyses. These include the low FODMAP diet, which shows the most consistent benefits for global symptoms, pain, and bloating among dietary strategies (Cuffe et al., 2025); brain–gut behavioral therapies – notably cognitive behavioral therapy and gut-directed hypnotherapy – which improve global symptoms and pain with durable effects and growing digital accessibility (Berry et al., 2023); and subtype-specific pharmacotherapy, such as secretagogues for IBS-C, 5-HT3 antagonists for IBS-D, and low-dose TCAs with selected antispasmodics for pain/global symptoms (Black et al., 2018).
A second tier comprises adjuncts with good—but generally shorter-term or moderate-certainty – evidence. Here, peppermint oil demonstrates short-term reductions in abdominal pain and improvements in global symptoms (Ingrosso et al., 2022), while soluble fiber (psyllium) improves stool form and some global outcomes; conversely, insoluble bran is often counterproductive and was avoided for many patients (Radziszewska et al., 2023).
Beyond these general measures, a third tier targets specific mechanisms increasingly recognized in IBS. Anorectal biofeedback is first-line when defecatory disorder coexists, supported by randomized trials and clinical guidelines (Rao et al., 2010). Thoracoabdominal motion biofeedback addresses abdominophrenic dyssynergia and visible distension, with placebo-controlled RCT evidence (Barba et al., 2024). Finally, HRV-guided/respiratory biofeedback aims to blunt stress reactivity and lessen symptom burden, with encouraging pilot RCTs (Exarchopoulou et al., 2024).
A fourth tier remains explicitly experimental. EEG neurofeedback shows preliminary promise for symptom severity and mood, but small samples and protocol heterogeneity preclude firm conclusions at present (Ebrahime et al., 2023).
Together, these tiers informed our complex, client-centred design: Level-1 modalities form the backbone for all participants; Level-2 options are added judiciously to address residual symptoms; Level-3 biofeedback modules are deployed when targeted mechanisms are identified; and Level-4 approaches are reserved for research contexts or individualized consideration. This layered strategy underpins the therapy described in the Methods and tested in the present study.
Rationale for a complex, integrated program
Given that single‑modality interventions often yield partial responses, an integrated, mechanism‑matched program may produce additive or synergistic benefits across symptom clusters and HRQoL domains. In practice, such a program can combine (i) dietitian‑led, evidence‑based nutrition (e.g., low‑FODMAP with structured reintroduction, or alternative diets when indicated), (ii) pharmacologic therapy tailored to subtype and pain, (iii) brain–gut behavioral treatment (CBT or GDH), and (iv) targeted biofeedback (anorectal for DD; thoracoabdominal for distension; HRV‑guided training for stress reactivity), with stepped‑care intensity and personalization by phenotype and mechanism. This aligns with guideline‑recommended care while directly addressing frequently neglected contributors – pelvic floor dyssynergia, abnormal respiratory–abdominal patterns, and dysregulated autonomic arousal – that drive symptom severity and quality‑of‑life impairment. (Lacy et al., 2021)
Study aim
To extend this literature, we evaluated pre‑/post‑intervention outcomes of a multimodal complex therapy, providing in our Center, incorporating biofeedback in 84 adults with IBS. We hypothesized clinically meaningful improvements in symptom severity and HRQoL, assessed with validated patient‑reported outcomes (e.g., IBS‑Symptom Severity Score [IBS‑SSS] and IBS‑QOL), and explored whether gains generalized across cardinal symptom domains (pain, bloating/distension, stool habit). (Francis et al., 1997)
Methods
Study Design
We conducted a single‐arm, pre–post observational study over 12 weeks to evaluate changes in symptom severity and health‐related quality of life (QoL) following a standardized, therapist-delivered intervention. Assessments were administered immediately before the first session (baseline) and after the twelfth session (post-treatment). Participants completed all outcome questionnaires at both time points. For descriptive and exploratory reporting, baseline severity strata (mild, moderate, severe) were defined a priori based on baseline symptom severity and were used only for subgroup summaries. The study was performed in accordance with the Declaration of Helsinki; all participants provided written informed consent. Analyses were conducted on complete cases (n=84); enrollment‑to‑completion drop‑out was 30.0% (36/120).
IBS-SSS (Primary Outcome)
Symptom severity was measured with the Irritable Bowel Syndrome–Symptom Severity Scale (IBS-SSS), comprising five items that assess abdominal pain intensity, abdominal pain frequency, abdominal distension, dissatisfaction with bowel habits, and interference with life. Items yield a 0–500 total score, with higher scores indicating greater symptom burden. The five item scores and the total score were analyzed at baseline and post-treatment. Subgroup summaries by baseline severity strata (mild/moderate/severe) were prespecified.
IBS-QoL (Secondary Outcome)
Health-related quality of life was assessed with the 34-item IBS-QOL. Each item is rated on a 5-point Likert scale (1–5). Following the scoring manual, items were reverse-coded so that larger values reflect better QoL.
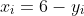
For any scale with KKK items (total or subscale) we report the item mean (1–5; higher = better)
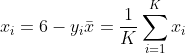
and a 0–100 transformed score
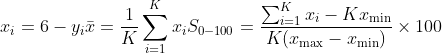
For a 1–5 scale this simplifies to
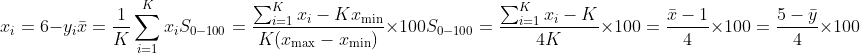
Higher scores indicate better QoL. Eight subscales were computed on the same 0–100 metric after reverse-coding: Dysphoria, Interference with Activity, Body Image, Health Worry, Food Avoidance, Social Reaction, Sexual, and Relationships. Pre/post totals and subscales were analyzed for the full sample; subgroup summaries used the same baseline severity strata.
Assessment of Autonomic Balance
Peripheral autonomic nervous system signals were recorded using the GP8 Amp 8-channel biofeedback amplifier and Alive Pioneer Plus software (Somatic Vision Inc., USA). The following parameters were acquired: heart rate (HR), heart rate variability (HRV), peripheral skin temperature, respiratory rate, skin conductance level (SCL), and surface electromyography (sEMG). HRV indices – including SDNN, total power, LF/HF ratio, stress index, sympathetic nervous system (SNS) index, and parasympathetic nervous system (PNS) index – were first derived in Alive Pioneer Plus and then exported for further processing in Kubios HRV Scientific/Lite (University of Eastern Finland). These recordings supported individualized titration of self-regulation targets (e.g., resonant-rate breathing) and were not used as primary endpoints.
Therapy Description (12 weeks; three stages; HRV/SCL/temperature biofeedback)
Overview and format
Participants received a 12‑week, one‑to‑one, client‑centred program grounded in a biopsychosocial model. Each weekly session lasted ~50–60 minutes and combined psychoeducation with autonomic self‑regulation using heart‑rate variability (HRV), skin conductance level (SCL/galvanic skin response), and peripheral temperature biofeedback. Between sessions, patients completed structured home practice: daily paced breathing at the individual’s resonant frequency, a brief symptom/trigger diary, and short behavioural tasks to generalize clinic gains to everyday contexts. Instrumentation included the GP8 Amp 8‑channel amplifier with Alive Pioneer Plus for acquisition and real‑time displays (e.g., RSA, LF/HF), with Kubios HRV used for analysis and calibration of training targets. Adherence and fidelity were monitored by attendance (0–12), weekly home‑practice review (minutes, diary completion), and therapist checklists. Pelvic‑floor/anorectal sEMG biofeedback was not delivered in this program.
Stage 1 – Autonomic preparation and early symptom relief (Weeks 1–4)
Aim. Establish a shared model of IBS (gut–brain/autonomic loops) and train patients to enter and sustain a “healthy HRV” state characterized by high respiratory sinus arrhythmia and high‑amplitude HRV at the individual resonant frequency, with smooth heart‑rhythm patterns. In parallel, shape SCL (from reactivity toward orienting) and finger temperature (vasomotor regulation).
Core procedures.
- Resonant‑frequency titration (clinic) followed by daily home practice (10–20 minutes) of paced breathing at resonance. (Shaffer & Meehan, 2020)
- Weekly variation of drills to prevent rapid habituation/“gaming” – tasks are adjusted in focus (breath, posture), cognitive load (simple tasks/game feedback), and context to maintain adaptive HRV/SCL/temperature responses.
- Targeted “tension‑focus” work: patients apply regulation to abdominal/postural musculature using Centre‑developed drills several times per day (including pre‑sleep) to reduce local guarding and visceral threat reactivity.
- Autonomic profile broadening: beyond breathing, incorporate techniques that reliably move HRV parameters, SCL and temperature toward targets (orienting vs fight/flight/freeze; warming cold extremities), acknowledging common vasomotor spasticity patterns in refractory IBS.
Outcome of Stage 1. Patients demonstrate stable HRV/SCL/temperature control across contexts, reduced baseline reactivity, and readiness for controlled symptom provocation.
Stage 2 – Symptom provocation with autonomic stabilization (Weeks 5–9)
Aim. Diminish abdominal pain, distress, and abnormal peristaltic responses by pairing controlled interoceptive provocation with on‑the‑spot autonomic stabilization learned in Stage 1.
Core procedures.
- Provocation targets. Therapist‑guided palpation of pre‑identified abdominal “tension foci” – typically near the proximal/distal colon or over abdominal sympathetic plexus regions – to elicit symptom‑linked stress signatures (fight/flight or freeze, the latter frequent in IBS).
- Real‑time regulation. Patients monitor HRV/SCL/temperature in real time and deploy regulation until autonomic targets are restored and regional pain diminishes.
- Repetition and generalization. The provoke‑then‑regulate sequence is repeated and distributed across foci and contexts, progressing from quiet to task‑loaded conditions (posture changes, mild multitasking) to consolidate extinction of conditioned responses and reduce reliance on avoidance.
Conceptual frame. This stage also introduces the working model of “functional cysts” –locally stabilized autonomic attractors in neural/tissue/organ networks (e.g., microcirculatory and enteric patterns) that can buffer or decouple global autonomic influences. Patients learn that persistent symptoms can reflect regional loops that require local provocation + global stabilization to unwind. Elements of desuggestion are used to reduce suggestibility‑driven symptom fluctuations.
Outcome of Stage 2. Marked reduction of core IBS symptoms (pain, distension, urgency) and improved volitional control when confronted with interoceptive threat cues; patients approach a “white period” (sustained low symptom burden) before advancing.
Stage 3 – Psychosocial triggers, consolidation, and relapse prevention (Weeks 10–12)
Aim. After a “white period” (symptoms sufficiently quiescent), identify and neutralize external psychosocial triggers that rapidly re‑evoke symptoms, convert skills into durable habits, and finalize a maintenance plan.
Core procedures.
- Trigger mapping and graded exposure. Using diaries, patients select high‑value real‑life contexts (e.g., stressful conversations, social/meal situations). They conduct graded in‑vivo or imaginal exposure while tracking HRV/SCL/temperature and using scripts that emphasize orienting and self‑efficacy over avoidance.
- Affective and cognitive coping strategies. Patients formalize coping scripts (attentional refocusing, expectancy testing/desuggestion strategies, behavioural options) that directly address interpersonal and environmental precipitants.
- Stress‑test and problem‑solve. Vary posture, pace, setting, and mild multitasking to stress‑test skill transfer; address barriers (time, environment, social support) and optimize home‑practice dose.
- Relapse prevention. A written plan details practice schedules, early‑warning signs, if‑then responses, and options for booster sessions.
Outcome of Stage 3. Skills are generalized across situations, psychosocial precipitants are demystified and manageable, and patients exit with a personalized maintenance plan.
Modality scope (for clarity)
This protocol purposefully does not include pelvic‑floor/anorectal sEMG biofeedback for dyssynergic defecation; such treatment is outside the present program’s scope. The active biofeedback modalities are HRV, SCL (GSR), and peripheral temperature, delivered with the instrumentation noted above.
Statistical Methods
Analyses were prespecified and two-tailed with α = 0.05. Descriptive statistics (n, mean, SD, median, IQR, range) were computed for all continuous variables. Paired t-tests compared pre/post means for IBS-SSS total and items, IBS-QoL total (item-mean and 0–100), and each IBS-QoL subscale; 95% CIs for mean differences were derived from the t distribution. Cohen’s dz (within-subject effect size) was reported for all paired comparisons. As a nonparametric confirmation, Wilcoxon signed-rank tests were also calculated; for subscales, p values were adjusted using Benjamini–Hochberg FDR across the eight tests. Subgroup analyses summarized within-group pre/post changes for the mild/moderate/severe strata and evaluated between-group differences in change via one-way ANOVA on change scores (with Kruskal–Wallis as a nonparametric complement); effect magnitude was indexed by η². Sensitivity analyses included outlier-excluded re-estimation (3×IQR rule on change scores) and 10% trimmed-mean differences. Exploratory analyses examined associations between ΔIBS-SSS and ΔIBS-QoL totals and subscales using Pearson and Spearman correlations (with Fisher’s z CIs for Pearson), and tested sex and age interactions with linear models including ΔSeverity × Sex and ΔSeverity × Age terms. Analyses were performed in Python (NumPy/SciPy/Pandas); reproducible code and outputs are available upon request.
Participants
Of 120 adults enrolled, 84 (70.0%) completed the 12-week program and both assessments; 36 (30.0%) withdrew. All completers contributed pre- and post-treatment data for both the primary outcome (IBS-SSS Total Symptom Severity) and the secondary outcome (IBS-QoL total), yielding 100% data completeness in the analytic sample (n = 84). The mean age was 34.4 ± 11.9 years, and 59/84 (70.2%) were female. At baseline, 16/84 (19.0%) were classified as mild, 11/84 (13.1%) as moderate, and 57/84 (67.9%) as severe. Baseline Total Symptom Severity averaged 307.7 ± 100.2 points, while baseline IBS-QoL total (0–100 scale; higher = better) averaged 28.9 ± 15.8. Detailed baseline characteristics overall and stratified by severity subgroup are shown in Table 1.
Table 1. Baseline characteristics
|
Characteristic |
Overall |
Mild |
Moderate |
Severe |
|
N |
84 |
16 |
11 |
57 |
|
Age, years – mean (SD) |
34.4 (11.9) |
28.7 (7.9) |
38.3 (12.9) |
35.2 (12.2) |
|
Age – median [IQR] |
32.0 [24.8, 41.2] |
26.5 [23.8, 32.8] |
37.0 [29.5, 48.5] |
32.0 [25.0, 42.0] |
|
Female – n (%) |
59 (70.2%) |
14 (87.5%) |
7 (63.6%) |
38 (66.7%) |
|
Male – n (%) |
25 (29.8%) |
2 (12.5%) |
4 (36.4%) |
19 (33.3%) |
|
Baseline Total Severity – mean (SD) |
307.7 (100.2) |
136.8 (15.4) |
234.6 (18.0) |
369.8 (37.8) |
|
Baseline QoL Total (0–100) – mean (SD) |
28.9 (15.8) |
49.2 (10.2) |
37.8 (16.5) |
21.5 (10.2) |
Notes: Total Symptom Severity is scored such that higher values indicate greater symptom burden; IBS-QoL totals are presented on a 0–100 transformed scale with higher values indicating better quality of life.
Symptom Severity (Total Score)
Across all participants (n = 84), Total Symptom Severity decreased markedly from 307.7 ± 100.2 pre-therapy to 137.0 ± 83.1 post-therapy. Distributional shifts are shown in Fig. 1-1.
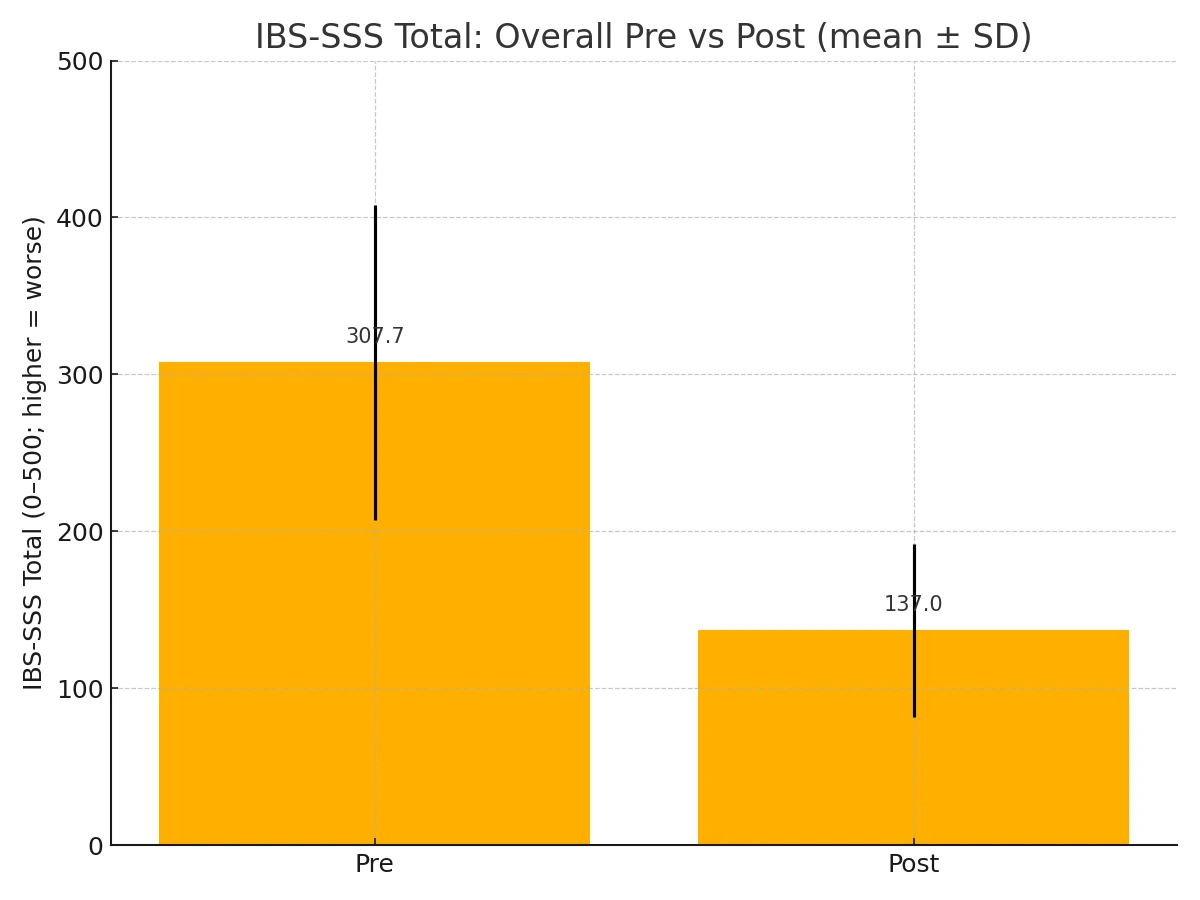
Figure 1-1. Total Symptom Severity pre vs post (overall).
Within each subgroup, Total Symptom Severity declined substantially from pre to post. SSS Total – by baseline severity subgroup (pre vs post; mean ± SD) (Fig. 1-2)
Mild (n=16): Pre 136.85 ± 15.39 → Post 65.14 ± 16.94
Moderate (n=11): Pre 234.56 ± 17.97 → Post 111.88 ± 21.08
Severe (n=57): Pre 369.84 ± 37.85 → Post 162.08 ± 45.66
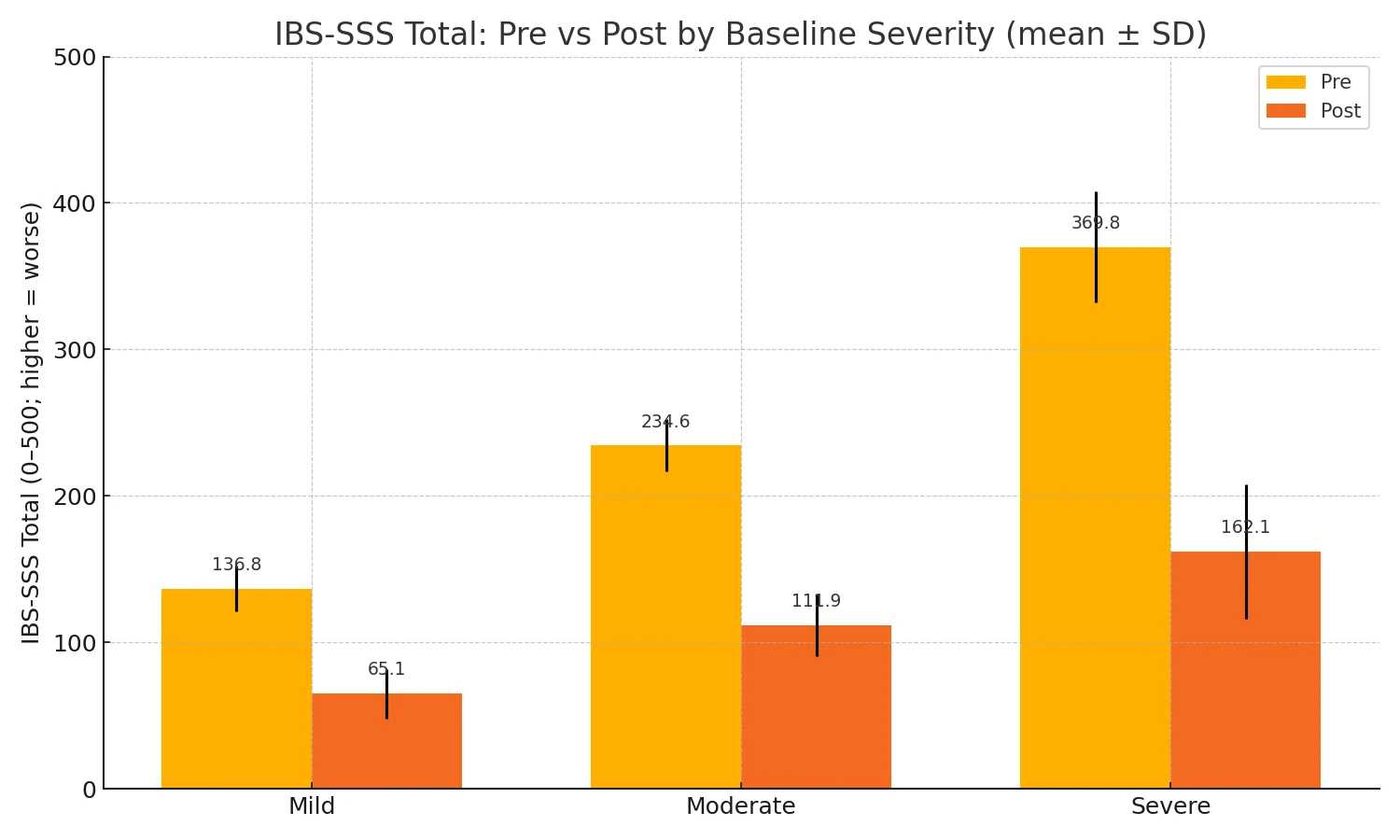
Figure 1-2. Total severity pre- and post- by base line severity.
All four comparisons show large within‑subject effects with highly significant paired t‑tests; Wilcoxon signed‑rank tests corroborate each result. The absolute improvement grows with baseline severity (largest in the severe group), consistent with greater headroom on a bounded 0–500 scale. (Table 2)
Table 2. Paired comparative statistics for IBS-SSS (0-500; higher = worse)
|
Group |
n |
Mean Δ (points) |
95% CI for Δ |
Cohen’s dz |
Paired t (df) |
p (t‑test) |
Wilcoxon p |
|
Overall |
84 |
−170.7 |
−186.7 to −154.7 |
−2.32 |
−21.25 (df=83) |
2.57×10⁻³⁵ |
1.71×10⁻¹⁵ |
|
Mild |
16 |
−71.7 |
−80.1 to −63.3 |
−4.57 |
−18.26 (df=15) |
1.17×10⁻¹¹ |
3.05×10⁻⁵ |
|
Moderate |
11 |
−122.7 |
−135.4 to −110.0 |
−6.50 |
−21.56 (df=10) |
1.03×10⁻⁹ |
9.77×10⁻⁴ |
|
Severe |
57 |
−207.8 |
−222.9 to −192.6 |
−3.64 |
−27.49 (df=56) |
3.37×10⁻³⁴ |
5.14×10⁻¹¹ |
Quality of Life (IBS-QoL)
QoL improved substantially. On the 0–100 total (sum of 34 matched items), the mean (n=84) increased from 28.93 ± 15.77 to 74.11 ± 7.48. (Fig. 2-1)
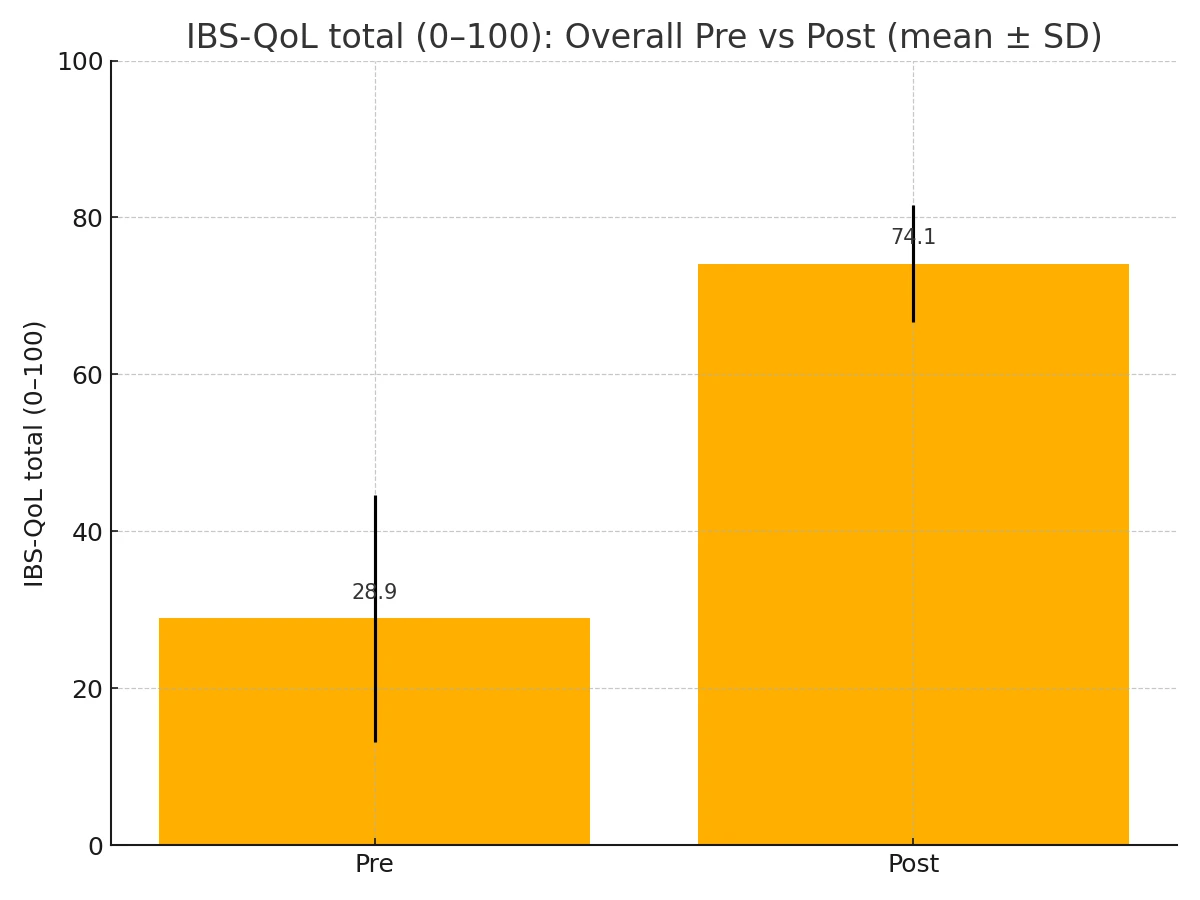
Figure 2-1. IBS-QoL Total, pre vs post (overall).
The improvements of QoL total score by baseline severity subgroup (pre vs post; mean ± SD) are shown in Fig. 2-2.
Mild: Pre 49.14 ± 10.19 → Post 81.30 ± 3.06 (n=16)
Moderate: Pre 37.83 ± 16.47 → Post 75.74 ± 5.41 (n=11)
Severe: Pre 21.54 ± 10.23 → Post 71.78 ± 7.38 (n=57)
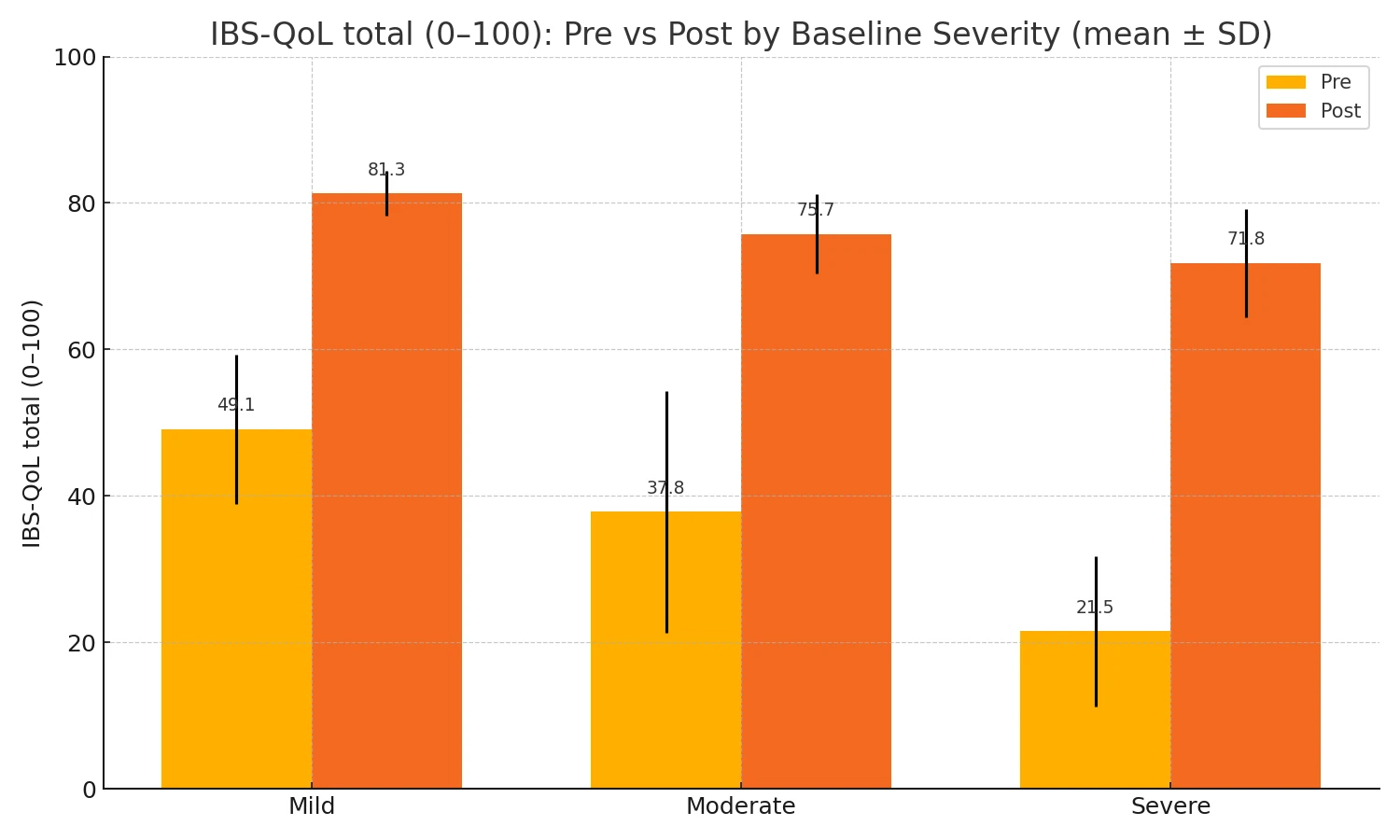
Figure 2-2. IBS-QoL Total pre- and post- by base line severity
The paired pre/post statistics for IBS‑QoL Total (0–100; higher = better) for all subgroups is shown in Table 3.
Table 3. Paired pre/post statistics for IBS‑QoL Total (0–100; higher = better)
|
Group |
n |
Mean Δ (post−pre) |
95% CI for Δ |
Cohen’s dz |
t (df) |
p (t‑test) |
Wilcoxon p |
|
Overall |
84 |
+45.17 |
+41.89 to +48.46 |
2.984 |
27.35 (df=83) |
2.80×10⁻⁴³ |
1.71×10⁻¹⁵ |
|
Mild |
16 |
+32.15 |
+25.68 to +38.62 |
2.647 |
10.59 (df=15) |
2.34×10⁻⁸ |
3.05×10⁻⁵ |
|
Moderate |
11 |
+37.90 |
+25.65 to +50.15 |
2.078 |
6.89 (df=10) |
4.23×10⁻⁵ |
1.00×10⁻³ |
|
Severe |
57 |
+50.23 |
+46.92 to +53.55 |
4.022 |
30.37 (df=56) |
1.80×10⁻³⁶ |
5.13×10⁻¹¹ |
All eight subscales showed significant pre–post gains with large within-subject effects. (Fig. 2-3)
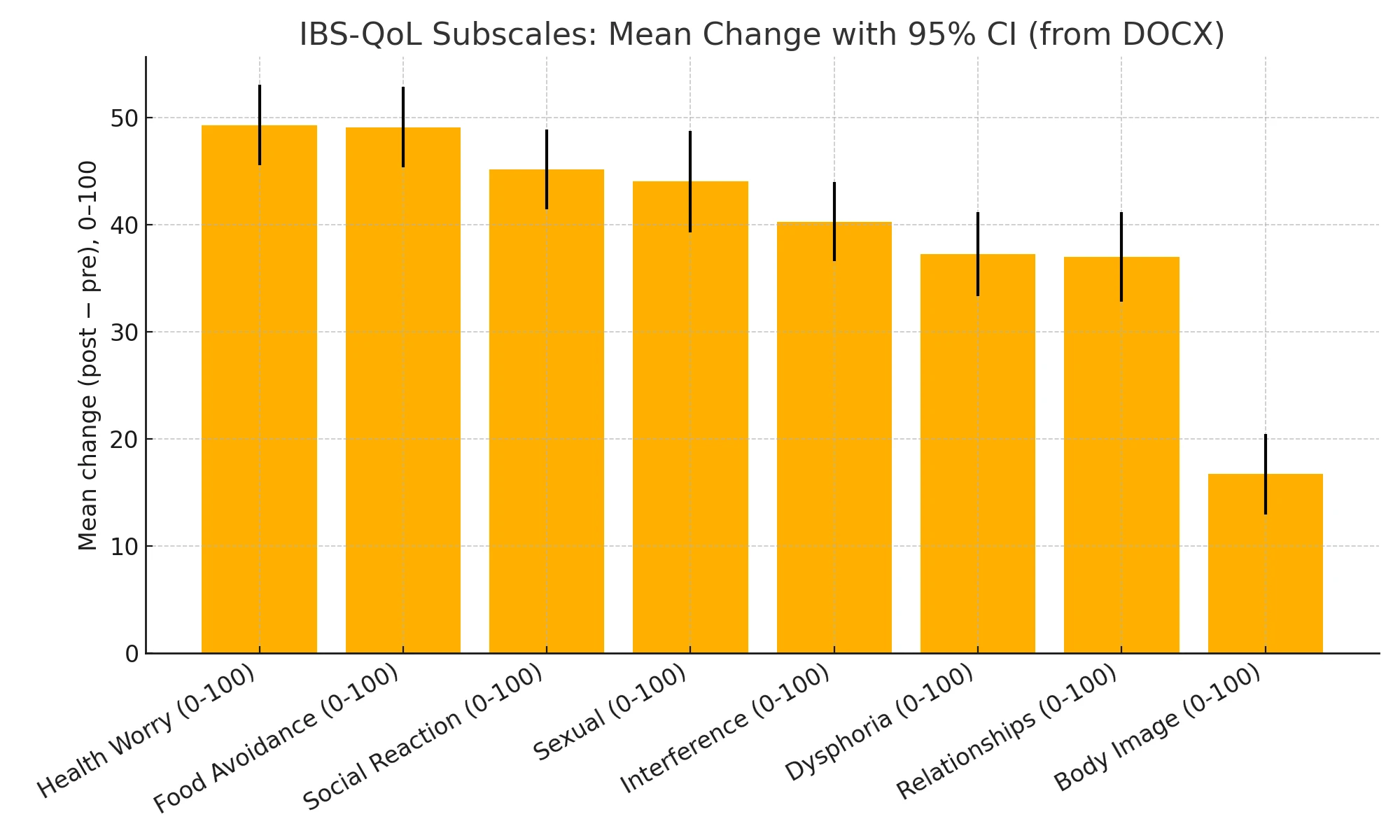
Figure 2-3. Mean Change (post-pre) for IBS-QoL Subscales.
All eight domains improved substantially and significantly after the program (q ≪ 0.001 across all subscales). The largest average gains were in Health Worry and Food Avoidance, followed by Social Reaction and Sexual function; Body Image improved the least. (Table 4.)
Table 4. Improvements
|
Subscale |
n |
Pre mean |
Post mean |
Mean Δ (post−pre) |
95% CI for Δ |
Cohen’s dz |
t(df) |
p (t‑test) |
q (BH‑FDR) |
|
Health Worry |
84 |
25.50 |
74.80 |
+49.30 |
+45.55 to +53.06 |
2.847 |
26.09 (83) |
9.25×10⁻⁴² |
4.78×10⁻⁴¹ |
|
Food Avoidance |
84 |
22.81 |
71.92 |
+49.10 |
+45.34 to +52.86 |
2.837 |
26.00 (83) |
1.19×10⁻⁴¹ |
4.78×10⁻⁴¹ |
|
Social Reaction |
84 |
28.05 |
73.21 |
+45.16 |
+41.45 to +48.87 |
2.642 |
24.21 (83) |
2.25×10⁻³⁹ |
6.00×10⁻³⁹ |
|
Sexual |
84 |
30.80 |
74.85 |
+44.04 |
+39.28 to +48.80 |
2.010 |
18.42 (83) |
4.63×10⁻³¹ |
6.18×10⁻³¹ |
|
Interference |
84 |
32.57 |
72.87 |
+40.31 |
+36.63 to +43.98 |
2.382 |
21.83 (83) |
3.82×10⁻³⁶ |
7.63×10⁻³⁶ |
|
Dysphoria |
84 |
25.11 |
62.38 |
+37.27 |
+33.33 to +41.21 |
2.055 |
18.83 (83) |
1.06×10⁻³¹ |
1.69×10⁻³¹ |
|
Relationships |
84 |
36.01 |
73.01 |
+37.00 |
+32.82 to +41.18 |
1.922 |
17.62 (83) |
8.80×10⁻³⁰ |
1.01×10⁻²⁹ |
|
Body Image |
84 |
32.06 |
48.80 |
+16.74 |
+12.99 to +20.49 |
0.969 |
8.88 (83) |
1.14×10⁻¹³ |
1.14×10⁻¹³ |
After Benjamini–Hochberg FDR correction across the eight subscales, all remained significant (q < .001). The largest average improvements were observed for Health Worry (Δ ≈ +49.3), Food Avoidance (Δ ≈ +49.1), and Social Reaction (Δ ≈ +45.2); the smallest for Body Image (Δ ≈ +16.7) – still substantial.
Exploratory Analyses
Across participants (n = 84), improvements in symptoms (more negative ΔSeverity) were moderately associated with gains in quality of life (more positive ΔQoL Total): Pearson r = −0.342 (95% CI −0.518 to −0.137), p = 0.00147; Spearman ρ = −0.333, p = 0.00198 (Figure 3). Subscale-level associations on the 0–100 scales showed a consistent negative pattern, ranging from −0.411 (Body Image, p = 1.1×10⁻4) to −0.21 (Health Worry, p = 0.0539); the remaining subscales were significant at p < 0.05 (Interference −0.347, Sexual −0.282, Social Reaction −0.308, Relationships −0.277, Food Avoidance −0.237, Dysphoria −0.225).
Figure 3. Association between change in IBS-SSS and change in IBS-QoL Total
In a multiple regression model predicting ΔQoL Total from ΔSeverity with sex and age as moderators (including main effects), the overall fit was modest (R² = 0.168, adj. R² = 0.115; n = 84). The ΔSeverity × Sex interaction was not significant (b = 0.0422, p = 0.472), indicating no evidence that the severity–QoL relationship differs by sex. The ΔSeverity × Age interaction showed a non-significant trend (b = 0.0030 per year, t=1.69; p = 0.0943), suggesting a slightly stronger coupling at older ages but not at conventional thresholds. The ΔSeverity main effect remained directionally consistent (b = −0.0997, p = 0.0587) after adding moderators.
Discussion
Principal findings
In this prospective cohort of 84 adults with IBS, we observed large, statistically and clinically significant improvements in both symptom severity and quality of life (QoL) following a multimodal (“complex”) therapeutic program that incorporated targeted biofeedback. Mean IBS total symptom severity decreased and QoL improved across all eight IBS‑QOL subscales – largest for Health Worry, Food Avoidance, and Social Reaction; smallest (but still substantial) for Body Image. Improvements were significant within each baseline severity stratum, with the largest absolute changes in those starting severe; between‑group differences in change were significant for both outcomes. Symptom improvement correlated only moderately with QoL gains (Pearson r≈−0.342), suggesting partially independent contributions of mechanisms targeted by the program.
Clinical significance of change
The magnitude of change comfortably exceeded accepted minimally clinically important differences (MCIDs). For IBS‑SSS, a ≥50‑point reduction is the standard individual‑level MCID; the mean reduction here was more than threefold that threshold. For IBS‑QOL, domain‑level MCIDs of ~10–14 points are commonly used; the observed subscale improvements (≈+16.7 to +49.3) surpassed those benchmarks.
How do these results compare with established single‑modality treatments?
Dietary therapy. Low‑FODMAP has the most consistent diet evidence. Contemporary randomized trials often report IBS‑SSS improvements around 100 points over ~6 weeks (e.g., −99.9 to −112.7), and responder rates of ~40–60% depending on endpoint and comparator. The mean symptom reduction in our cohort is larger, which is not unexpected given multimodal treatment and a pre–post (uncontrolled) design. (Eswaran et al., 2016)
Brain–gut behavioral therapy. Large RCTs (e.g., ACTIB) show that CBT produces between‑group advantages versus treatment‑as‑usual (TAU) of ≈35–62 IBS‑SSS points at 12 months, with 63–71% achieving ≥50‑point reductions. Those net effects – derived from controlled comparisons – are smaller in magnitude than our within‑subject pre–post changes, but they underscore the durability and clinical value of CBT components embedded in integrated care. (Everitt et al., 2019)
Gut‑directed hypnotherapy (GDH). GDH yields improvements comparable to low‑FODMAP in randomized trials, and systematic reviews support benefit on global symptoms. Uncontrolled series frequently report IBS‑SSS decreases on the order of 100–130 points – again smaller than our aggregate pre–post change but directionally consistent. (Peters et al., 2016) Pharmacological therapy. Network meta‑analyses rank 5‑HT3_33 antagonists (e.g., alosetron/ramosetron) among the most effective options for IBS‑D; secretagogues (e.g., linaclotide/plecanatide/lubiprostone) are effective for IBS‑C; low‑dose tricyclics and selected antispasmodics help with pain/global symptoms. However, drug trials typically report modest to moderate effect sizes and responder deltas versus placebo, reflecting both genuine drug effects and the high placebo response in IBS. The larger pre–post changes we observed likely reflect multi‑mechanism targeting plus design differences (no control). (Black et al., 2020)
Mechanistic interpretation
IBS reflects dysregulation across the microbiota–gut–brain axis, autonomic imbalance, altered motor/sensory patterns (e.g., dyssynergic defecation, abdominophrenic dyssynergia), and psychosocial factors. An integrated program can act on complementary levers: dietary modification to reduce luminal triggers; pharmacotherapy tailored to stool phenotype/pain; CBT/GDH to reduce GI‑specific anxiety and catastrophizing; pelvic‑floor biofeedback to restore defecatory coordination; and thoracoabdominal biofeedback to retrain breathing/abdominal wall use. The moderate coupling between symptom and QoL change (r≈−0.31) implies that non‑symptom mechanisms (e.g., reduced health worry, normalization of social/food behaviors, improved self‑efficacy) made independent contributions to QoL in this cohort.
Subgroup patterns and baseline severity
Participants beginning in the severe range (>300 on IBS‑SSS) showed the largest absolute reductions, moving the post‑treatment cohort mean into the mild range—consistent with “headroom” effects and the expected behavior of a bounded scale. That said, significant differences in change across baseline strata should be interpreted with caution due to regression to the mean and mathematical coupling between baseline and change scores, especially in uncontrolled designs. (Tunali et al., 2024)
Strengths and limitations
Strengths include comprehensive pre/post assessment using validated instruments, 100% retention, and convergence of effects across total severity, item‑level severity, QoL totals, and QoL subscales. Limitations include the absence of a control arm (susceptible to expectancy effects, secular trends, and regression to the mean), the inflation of within‑subject effect sizes (d<sub>z</sub>) relative to between‑group effects seen in RCTs, and the inability to apportion benefit among components (diet, CBT/GDH, pharmacotherapy, biofeedback). The unusually high retention may reflect program feasibility and patient engagement but also raises the possibility of selection factors that limit generalizability. Finally, although multiple endpoints moved in the expected direction with large effect sizes, the program’s duration and component dosing were not formally optimized or compared. (Barberio et al., 2022)
Comparison with integrated‑care literature
Our findings are congruent with the emerging consensus – and empirical data – that integrated, mechanism‑matched care outperforms single‑modality approaches for many patients with IBS. Narrative and empirical reviews advocate combining dietary, behavioral, and pharmacologic strategies within coordinated care; clinical programs report higher global improvement rates with integrated models than with usual care. The present outcomes extend this literature by suggesting that adding targeted biofeedback to address autonomic dis-balance may yield particularly strong gains in Food Avoidance, Social Reaction, and Health Worry – domains not uniformly addressed by diet or drugs alone. (Chey et al., 2021)
Conclusions
In this single‑arm, pre–post study of 84 adults with IBS, a 12‑week, three‑stage, client‑centred program – combining psychoeducation with HRV / SCL / temperature biofeedback, targeted interoceptive exposure with real‑time autonomic stabilization, and psychosocial trigger work – was associated with large and statistically significant improvements in both symptom severity and health‑related quality of life. Mean IBS‑SSS fell by 170.7 points (95% CI −186.7 to −154.7; d<sub>z</sub>=2.32), and IBS‑QOL improved markedly on both the 0–100 total and the item‑mean scale. Gains were robust across baseline severity strata, with the largest absolute reductions among participants who entered in the severe range, and retention was 70%, underscoring feasibility.
Quality‑of‑life benefits were broad‑based – all eight IBS‑QOL subscales improved – and were most pronounced for Health Worry, Food Avoidance, and Social Reaction, domains that are not reliably addressed by diet or pharmacotherapy alone. The moderate correlation between symptom change and QoL change (Pearson r≈−0.342) suggests that autonomic self‑regulation and psychosocial skills contributed independent patient‑centred gains beyond pain and bowel habit alone.
Taken together, these findings support the clinical promise of integrated, mechanism‑matched care for IBS: a staged protocol that (1) builds autonomic control at an individualized resonant frequency, (2) pairs provocation of abdominal “tension foci” with on‑the‑spot regulation to extinguish conditioned responses, and (3) consolidates skills in real‑life trigger contexts with relapse‑prevention planning. While pelvic‑floor/anorectal sEMG biofeedback was not part of this program, the present results indicate that autonomic‑focused biofeedback can be a useful adjunct alongside guideline‑concordant diet, pharmacotherapy, and brain–gut behavioral treatment within coordinated care pathways.
Limitations – including the absence of a control arm, potential expectancy and regression‑to‑the‑mean effects, and the inability to apportion benefit among components—warrant cautious interpretation. Nevertheless, the internal consistency of effects across total severity, item‑level severity, overall QoL, and QoL subscales, together with complete follow‑up, argue for signal credibility and program feasibility.
Implications and next steps. For practice, a pragmatic take‑home is that structured autonomic training plus contextualized exposure and coping can meaningfully improve outcomes for many patients and may be prioritized when health worry, food avoidance, and social reactivity dominate the burden. For research, a randomized, controlled trial is now justified to test the incremental value of autonomic biofeedback within integrated care, to examine durability, and to evaluate mechanistic markers alongside patient‑reported outcomes.
References
Ahadi, T., Madjlesi, F., Mahjoubi, B., Mirzaei, R., Forogh, B., Daliri, S. S., Derakhshandeh, S. M., Behbahani, R. B., & Raissi, G. R. (2014). The effect of biofeedback therapy on dyssynergic constipation in patients with or without Irritable Bowel Syndrome. Journal of research in medical sciences: the official journal of Isfahan University of Medical Sciences, 19(10), 950–955.
Alborzi Avanaki, F., Rafiee, S., Mofid, A., & Alborzi, A. (2023). Biofeedback Treatment Can Improve Clinical Condition and Quality of Life in Patients with Pelvic Floor Dyssynergy with Irritable Bowel Syndrome: A Prospective Cohort Study. Middle East Journal of Digestive Diseases, 15(1), 45–52.
Allied Digestive Health. (2023). Lifestyle Changes to Help IBS. Retrieved from https://allieddigestivehealth.com/lifestyle-changes-to-help-ibs/
Bancroft, W. J. (1976). Suggestology and Suggestopedia: The Theory of the Lozanov Method.
Barba, E., Burri, E., Accarino, A., Malagelada, C., Rodriguez-Urrutia, A., Soldevilla, A., Malagelada, J.-R., & Azpiroz, F. (2015). Biofeedback-guided control of abdominothoracic muscular activity reduces regurgitation episodes in patients with rumination. Clinical Gastroenterology and Hepatology, 13(1), 100-106.e1. https://doi.org/10.1016/j.cgh.2014.04.018
Barba, E., Livovsky, D. M., Accarino, A., & Azpiroz, F. (2024). Thoracoabdominal Wall Motion-Guided Biofeedback Treatment of Abdominal Distention: A Randomized Placebo-Controlled Trial. Gastroenterology, 167(3), 538–546.e1. https://doi.org/10.1053/j.gastro.2024.03.005
Barberio, B., Savarino, E. V., Black, C. J., & Ford, A. C. (2022). Placebo response rates in trials of licensed drugs for irritable bowel syndrome with constipation or diarrhea: Meta-analysis. Clinical Gastroenterology and Hepatology, 20(5), e923–e944. https://doi.org/10.1016/j.cgh.2021.05.025
Bassotti, G., & Whitehead, W. E. (1994). Biofeedback as a treatment approach to gastrointestinal tract disorders. American Journal of Gastroenterology, 89(2), 158–164.
Bassotti, G., & Whitehead, W. E. (1997). Biofeedback, relaxation training, and cognitive behaviour modification as treatments for lower functional gastrointestinal disorders. QJM: An International Journal of Medicine, 90(8), 545–550. https://doi.org/10.1093/qjmed/90.8.545
Berry, S. K., Berry, R., Recker, D., Botbyl, J., Pun, L., & Chey, W. D. (2023). A Randomized Parallel-group Study of Digital Gut-directed Hypnotherapy vs Muscle Relaxation for Irritable Bowel Syndrome. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association, 21(12), 3152–3159.e2. https://doi.org/10.1016/j.cgh.2023.06.015
Biniszewska, O., Jacenik, D., Tarasiuk, A., & Fichna, J. (2024). Current and future pharmacotherapies for the management of constipation-predominant irritable bowel syndrome. Expert Opinion on Pharmacotherapy, 25(8), 1039–1049. https://doi.org/10.1080/14656566.2024.2366993
Black, C. J., & Ford, A. C. (2020). Global burden of irritable bowel syndrome: Trends, predictions and risk factors. Nature Reviews Gastroenterology & Hepatology, 17, 473–486. https://doi.org/10.1038/s41575-020-0286-8
Black, C. J., Burr, N. E., Camilleri, M., Earnest, D. L., Quigley, E. M., Moayyedi, P., Houghton, L. A., & Ford, A. C. (2020). Efficacy of pharmacological therapies in patients with IBS with diarrhoea or mixed stool pattern: systematic review and network meta-analysis. Gut, 69(1), 74–82. https://doi.org/10.1136/gutjnl-2018-318160
Black, C. J., Burr, N. E., Quigley, E. M. M., Moayyedi, P., Houghton, L. A., & Ford, A. C. (2018). Efficacy of Secretagogues in Patients With Irritable Bowel Syndrome With Constipation: Systematic Review and Network Meta-analysis. Gastroenterology, 155(6), 1753–1763. https://doi.org/10.1053/j.gastro.2018.08.021
Black, C. J., Staudacher, H. M., & Ford, A. C. (2022). Efficacy of a low FODMAP diet in irritable bowel syndrome: Systematic review and network meta-analysis. Gut, 71(6), 1117–1126. https://doi.org/10.1136/gutjnl-2021-325214
Black, C. J., Thakur, E. R., Houghton, L. A., Quigley, E. M. M., & Ford, A. C. (2020). Efficacy of psychological therapies for irritable bowel syndrome: Systematic review and network meta-analysis. Gut, 69(8), 1441–1451. https://doi.org/10.1136/gutjnl-2020-321191
Blanchard, E. B. (2001). Irritable bowel syndrome: Psychosocial assessment and treatment. American Psychological Association. https://doi.org/10.1037/10393-000
Cadeddu, F., Salis, F., De Luca, E., Ciangola, I., & Milito, G. (2015). Efficacy of biofeedback plus transanal stimulation in the management of pelvic floor dyssynergia: a randomized trial. Techniques in Coloproctology, 19(6), 333–338. doi:10.1007/s10151-015-1292-7
Canavan, C., West, J., & Card, T. (2014). The epidemiology of irritable bowel syndrome. Clinical Epidemiology, 6, 71–80. https://doi.org/10.2147/CLEP.S40245
Chandar, A. K. (2017). Diagnosis and treatment of irritable bowel syndrome with predominant constipation in the primary-care setting: focus on linaclotide. International Journal of General Medicine, 10, 385–393. https://doi.org/10.2147/IJGM.S126581
Chey, W. D., Keefer, L., Whelan, K., & Gibson, P. R. (2021). Behavioral and diet therapies in integrated care for patients with irritable bowel syndrome. Gastroenterology, 160(1), 47–62. https://doi.org/10.1053/j.gastro.2020.07.054
Chiarioni, G., & Whitehead, W. E. (2008). The role of biofeedback in the treatment of gastrointestinal disorders. Nature Clinical Practice Gastroenterology & Hepatology, 5(7), 371–382. doi:10.1038/ncpgasthep1150
Chiarioni, G., Whitehead, W. E., Pezza, V., Morelli, A., & Bassotti, G. (2006). Biofeedback Is Superior to Laxatives for Normal Transit Constipation Due to Pelvic Floor Dyssynergia. Gastroenterology, 130(3), 657–664. doi:10.1053/j.gastro.2005.11.014
Comparative efficacy and safety of probiotics for the treatment of irritable bowel syndrome: a systematic review and network meta-analysis protocol. (2019). BMJ Open, 9(12), e032018.
Cuffe, M.S., Staudacher, H.M., Aziz, I. et al. (6 more authors) (2025) Efficacy of dietary interventions in irritable bowel syndrome: a systematic review and network meta-analysis. The Lancet Gastroenterology & Hepatology, 10 (6). pp. 520-536. ISSN 2468-1253 https://doi.org/10.1016/s2468-1253(25)00054-8
DeGuire, S., Gevirtz, R., Hawkinson, D., & Dixon, K. (1996). Breathing retraining: A three-year follow-up study of treatment for hyperventilation syndrome and associated functional cardiac symptoms. Biofeedback and Self-Regulation, 21(2), 191–198. https://doi.org/10.1007/BF02284695
Dobbin, A., Dobbin, J., Ross, S. C., Graham, C., & Ford, M. J. (2013). Randomised controlled trial of brief intervention with biofeedback and hypnotherapy in patients with refractory irritable bowel syndrome. Journal of the Royal College of Physicians of Edinburgh, 43, 15–23. https://doi.org/10.4997/JRCPE.2013.104
Drossman, D. A., & Hasler, W. L. (2016). Rome IV—Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology, 150(6), 1257–1261. doi:10.1053/j.gastro.2016.03.035
Duncanson, K., Tikhe, D., Williams, G. M., & Talley, N. J. (2023). Irritable bowel syndrome – controversies in diagnosis and management. Expert Review of Gastroenterology & Hepatology, 17(7), 649–663. https://doi.org/10.1080/17474124.2023.2223975
Ekholm, M., Krouwels, M., & Knittle, K. (2024). Examining interactions of illness perceptions, avoidance behavior and patient status in predicting quality of life among people with irritable bowel syndrome. Health Psychology and Behavioral Medicine, 12(1), 2311986. https://doi.org/10.1080/21642850.2024.2311986
Ellen Johanne Vara, Jørgen Valeur, Trygve Hausken & Gülen Arslan Lied. (2016) Extra-intestinal symptoms in patients with irritable bowel syndrome: related to high total IgE levels and atopic sensitization?. Scandinavian Journal of Gastroenterology 51:8, pages 908-913.
Enck, P., Aziz, Q., Barbara, G., Farmer, A. D., Fukudo, S., Mayer, E. A., Niesler, B., Quigley, E. M., Rajilić-Stojanović, M., Schemann, M., et al. (2016). Irritable bowel syndrome. Nature Reviews Disease Primers, 2, 16014. https://doi.org/10.1038/nrdp.2016.14
Eswaran, S. L., Chey, W. D., Han-Markey, T., Ball, S., & Jackson, K. (2016). A Randomized Controlled Trial Comparing the Low FODMAP Diet vs. Modified NICE Guidelines in US Adults with IBS-D. The American journal of gastroenterology, 111(12), 1824–1832. https://doi.org/10.1038/ajg.2016.434
Everitt, H. A., Landau, S., O'Reilly, G., Sibelli, A., Hughes, S., Windgassen, S., Holland, R., Little, P., McCrone, P., Bishop, F., Goldsmith, K., Coleman, N., Logan, R., Chalder, T., Moss-Morris, R., & ACTIB trial group (2019). Assessing telephone-delivered cognitive-behavioural therapy (CBT) and web-delivered CBT versus treatment as usual in irritable bowel syndrome (ACTIB): a multicentre randomised trial. Gut, 68(9), 1613–1623. https://doi.org/10.1136/gutjnl-2018-317805
Exarchopoulou, K., Papageorgiou, A., Bacopoulou, F., Malisiova, E. K., Vlachakis, D., Chrousos, G. P., & Darviri, C. (2021). A Biofeedback-Assisted Stress Management Program for Patients with Irritable Bowel Syndrome: a Randomised Controlled Trial. EMBnet.journal, 26, e980. https://doi.org/10.14806/ej.26.1.980
Ford, A. C. (2024). Concordance between Rome III and Rome IV criteria in irritable bowel syndrome. Indian Journal of Gastroenterology, 43, 1079–1081. https://doi.org/10.1007/s12664-024-01624-z
Ford, A. C., Bercik, P., Morgan, D. G., Bolino, C., Pintos-Sanchez, M. I., & Moayyedi, P. (2013). Characteristics of functional bowel disorder patients: a cross-sectional survey using the Rome III criteria. Alimentary Pharmacology & Therapeutics, 39(3), 312–321. doi:10.1111/apt.12573
Ford, A. C., Lacy, B. E., & Talley, N. J. (2017). Irritable Bowel Syndrome. New England Journal of Medicine, 376(26), 2566–2578. doi:10.1056/nejmra1607547
Ford, A. C., Moayyedi, P., Chey, W. D., Harris, L. A., Lacy, B. E., Saito, Y. A., & Quigley, E. M. M. (2018). American College of Gastroenterology monograph on management of irritable bowel syndrome. American Journal of Gastroenterology, 113(6), S1–S18. https://doi.org/10.1038/s41395-018-0084-x
Fragkos, K. C. (2017). Spotlight on eluxadoline for the treatment of patients with irritable bowel syndrome with diarrhea. Clinical and Experimental Gastroenterology, 10, 229–240. https://doi.org/10.2147/CEG.S123621
Francis, C. Y., Morris, J., & Whorwell, P. J. (1997). The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Alimentary pharmacology & therapeutics, 11(2), 395–402. https://doi.org/10.1046/j.1365-2036.1997.142318000.x
Gao, Y., Chen, C., Huang, X., Liu, Y., Zhou, Z., & Pan, Y. (2025). Omentin-1, a protective adipokine for irritable bowel syndrome. Journal of Inflammation Research, 18, 1689–1701. https://doi.org/10.2147/JIR.S499613
Gaylord, S. A., Palsson, O. S., Garland, E. L., Faurot, K. R., Coble, R. S., Mann, D. J., Frey, W., Leniek, K., & Whitehead, W. E. (2011). Mindfulness training reduces the severity of irritable bowel syndrome in women: Results of a randomized controlled trial. American Journal of Gastroenterology, 106(9), 1678–1688. https://doi.org/10.1038/ajg.2011.184
Goldenberg, J. Z., Brignall, M., Hamilton, M., Beardsley, J., Batson, R. D., Hawrelak, J., Lichtenstein, B., & Johnston, B. C. (2019). Biofeedback for treatment of irritable bowel syndrome. The Cochrane database of systematic reviews, 2019(11), CD012530. https://doi.org/10.1002/14651858.CD012530.pub2
Haghbin, H., Hasan, F., Gangwani, M. K., Zakirkhodjaev, N., Lee-Smith, W., Beran, A., Kamal, F., Hart, B., & Aziz, M. (2024). Efficacy of dietary interventions for irritable bowel syndrome: A systematic review and network meta-analysis. Journal of Clinical Medicine, 13(24), 7531. https://doi.org/10.3390/jcm13247531
Hasan, S. S., Pearson, J. S., Morris, J., & Whorwell, P. J. (2019). SKYPE HYPNOTHERAPY FOR IRRITABLE BOWEL SYNDROME: Effectiveness and comparison with face-to-face treatment. International Journal of Clinical and Experimental Hypnosis, 67(1), 69–80. https://doi.org/10.1080/00207144.2018.1508759
Heymen, S., Jones, K. R., Scarlett, Y., & Whitehead, W. E. (2003). Biofeedback Treatment of Constipation. Diseases of the Colon & Rectum, 46(9), 1208–1217. doi:10.1007/s10350-004-6717-8
Hite, M., & Curran, T. (2020). Biofeedback for Pelvic Floor Disorders. Clinics in Colon and Rectal Surgery. doi:10.1055/s-0040-1714287
Hjelland, I. E., Svebak, S., Berstad, A., & Hausken, T. (2007). Breathing exercises with vagal biofeedback may benefit patients with functional dyspepsia. Scandinavian Journal of Gastroenterology, 42(9), 1054–1062. https://doi.org/10.1080/00365520701259208
Ingrosso, M. R., Ianiro, G., Nee, J., Lembo, A. J., Moayyedi, P., Black, C. J., & Ford, A. C. (2022). Systematic review and meta-analysis: efficacy of peppermint oil in irritable bowel syndrome. Alimentary pharmacology & therapeutics, 56(6), 932–941. https://doi.org/10.1111/apt.17179
Khademi, M., Ebrahimi, A., Nasiri Dehsorkhi, S., Daghaqzadeh, H., Nasiri Desorkhi, H., & Sadr Ameli, S. (2023). The effectiveness of neurofeedback on gastrointestinal symptoms, depression, anxiety, stress and quality of life in patients with irritable bowel syndrome: A randomized clinical trial with a control group. Journal of Isfahan Medical School, 41(712), 172–179. https://doi.org/10.48305/jims.v41.i712.0172
Koutsomanis, D., Lennard-Jones, J. E., Roy, A. J., & Kamm, M. A. (1995). Controlled randomised trial of visual biofeedback versus muscle training without a visual display for intractable constipation. Gut, 37(1), 95–99. doi:10.1136/gut.37.1.95
Lacy, B. E., Pimentel, M., Brenner, D. M., Chey, W. D., Keefer, L. A., Long, M. D., & Moshiree, B. (2021). ACG Clinical Guideline: Management of Irritable Bowel Syndrome. American Journal of Gastroenterology, 116(1), 17–44. DOI: 10.14309/ajg.0000000000001036
Lehrer, P. M., & Gevirtz, R. (2014). Heart rate variability biofeedback: how and why does it work? Frontiers in Psychology, 5, 756. https://doi.org/10.3389/fpsyg.2014.00756
Lehrer, P. M., Vaschillo, E., & Vaschillo, B. (2000). Resonant frequency biofeedback training to increase cardiac variability: Rationale and manual for training. Applied Psychophysiology and Biofeedback, 25(3), 177–191. https://doi.org/10.1023/A:1009554825745
Lesbros-Pantoflickova, D., Michetti, P., Fried, M., Beglinger, C., & Blum, A. L. (2004). Meta-analysis: The treatment of irritable bowel syndrome. Alimentary Pharmacology & Therapeutics, 20(12), 1253–1269. https://doi.org/10.1111/j.1365-2036.2004.02267.x
Li, C., Ying, Y., Zheng, Y., Li, X., & Lan, L. (2024). Epidemiology of irritable bowel syndrome: a systematic review and meta-analysis. Journal of Public Health and Epidemiology, 2(1), 1–20.
Longstreth, G. F., Thompson, W. G., Chey, W. D., Houghton, L. A., Mearin, F., & Spiller, R. C. (2006). Functional bowel disorders. Gastroenterology, 130(5), 1480–1491. https://doi.org/10.1053/j.gastro.2005.11.061
Lovell, R. M., & Ford, A. C. (2012). Effect of gender on prevalence of irritable bowel syndrome in the community: Systematic review and meta-analysis. American Journal of Gastroenterology, 107(7), 991–1000. https://doi.org/10.1038/ajg.2012.131
Mahmoudi, L., Shafiekhani, M., Dehghanpour, H., & Niknam, R. (2019). Community pharmacists’ knowledge, attitude, and practice of Irritable Bowel Syndrome (IBS): The impact of training courses. Advances in Medical Education and Practice, 10, 427–436. https://doi.org/10.2147/AMEP.S201904
Mayer, E. A., & Tillisch, K. (2011). The brain-gut axis in abdominal pain syndromes. Annual Review of Medicine, 62, 381–396. https://doi.org/10.1146/annurev-med-012309-103958
Mayer, E. A., Tillisch, K., & Gupta, A. (2015). Gut/brain axis and the microbiota. Journal of Clinical Investigation, 125(3), 926–938. doi:10.1172/jci76304
Mayo Clinic. (2017, March 28). The role of lifestyle-related treatments for IBS. Mayo Clinic. https://www.mayoclinic.org/medical-professionals/digestive-diseases/news/the-role-of-lifestyle-related-treatments-for-ibs/mac-20431272
Mayo Clinic. (2024). Irritable bowel syndrome - Diagnosis and treatment. Retrieved from https://www.mayoclinic.org/diseases-conditions/irritable-bowel-syndrome/diagnosis-treatment/drc-20360064
Meleine, M., & Matricon, J. (2014). Gender-related differences in irritable bowel syndrome: Potential mechanisms of sex hormones. World Journal of Gastroenterology, 20(22), 6725–6743. https://doi.org/10.3748/wjg.v20.i22.6725
Monash University. (n.d.). Mental health and IBS - Effectiveness of psychological treatments in IBS. Retrieved from https://www.monashfodmap.com/blog/mental-health-and-ibs-effectiveness-psychological-treatments-ibs/
Mousavi, T., Nikfar, S., & Abdollahi, M. (2020). An update on efficacy and safety considerations for the latest drugs used to treat irritable bowel syndrome. Expert Opinion on Drug Metabolism & Toxicology, 16(7), 583–604. https://doi.org/10.1080/17425255.2020.1767067
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). (2017). Eating, Diet, & Nutrition for Irritable Bowel Syndrome. Retrieved from https://www.niddk.nih.gov/health-information/digestive-diseases/irritable-bowel-syndrome/eating-diet-nutrition
NYU Langone Health. (n.d.). Medication for Irritable Bowel Syndrome. Retrieved from https://nyulangone.org/conditions/irritable-bowel-syndrome/treatments/medication-for-irritable-bowel-syndrome
Oka, P., Parr, H., Barberio, B., Black, C. J., Savarino, E. V., & Ford, A. C. (2020). Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. The Lancet Gastroenterology & Hepatology. doi:10.1016/s2468-1253(20)30217-x
Özdener, A. E., & Rivkin, A. (2017). Eluxadoline in the treatment of diarrhea-predominant irritable bowel syndrome. Drug Design, Development and Therapy, 11, 2827–2840. https://doi.org/10.2147/DDDT.S127405
Palsson, O. S., & Whitehead, W. E. (2013). Psychological treatments in functional gastrointestinal disorders: A primer for the gastroenterologist. Clinical Gastroenterology and Hepatology, 11(3), 208–216. https://doi.org/10.1016/j.cgh.2012.10.031
Patcharatrakul, T., & Gonlachanvit, S. (2011). Outcome of Biofeedback Therapy in Dyssynergic Defecation Patients With and Without Irritable Bowel Syndrome. Journal of Clinical Gastroenterology, 45(7), 593–598. doi:10.1097/mcg.0b013e31820c6001
Patcharatrakul, T., Valestin, J., Schmeltz, A., Schulze, K., & Rao, S. S. C. (2018). Factors Associated With Response to Biofeedback Therapy for Dyssynergic Defecation. Clinical Gastroenterology and Hepatology, 16(5), 715–721. doi:10.1016/j.cgh.2017.10.027
Patel, S., Doerfler, B., Boutros, K., Ng, S., Manuel, M., & DeSimone, E. (2021). Review of treatment options for irritable bowel syndrome with constipation and chronic idiopathic constipation. International Journal of General Medicine, 14, 1457–1468. https://doi.org/10.2147/IJGM.S274568
Patrick, D. L., Drossman, D. A., Frederick, I. O., DiCesare, J., & Puder, K. L. (1998). Quality of life in persons with irritable bowel syndrome: Development and validation of a new measure. Digestive Diseases and Sciences, 43(2), 400–411. https://doi.org/10.1023/A:1018831127942
Payne, S. (2004). Sex, gender, and irritable bowel syndrome: making the connections. Gender Medicine, 1(1), 18–28. doi:10.1016/s1550-8579(04)80007-x
Peters, S. L., Yao, C. K., Philpott, H., Yelland, G. W., Muir, J. G., & Gibson, P. R. (2016). Randomised clinical trial: the efficacy of gut-directed hypnotherapy is similar to that of the low FODMAP diet for the treatment of irritable bowel syndrome. Alimentary pharmacology & therapeutics, 44(5), 447–459. https://doi.org/10.1111/apt.13706
Poulsen, C. H., Eplov, L. F., Hjorthøj, C., Eliasen, M., Ebstrup, J. F., & Skovbjerg, S. (2016). Gastrointestinal symptoms related to the irritable bowel syndrome – a longitudinal population-based register study. Scandinavian Journal of Gastroenterology, 51(4), 420–426. https://doi.org/10.3109/00365521.2015.1117652
Poulsen, C. H., Eplov, L. F., Hjorthøj, C., Eliasen, M., Skovbjerg, S., & Dantoft, T. M. (2017). Irritable bowel symptoms and the development of common mental disorders and functional somatic syndromes identified in secondary care – a long-term, population-based study. Clinical Epidemiology, 9, 393–402. https://doi.org/10.2147/CLEP.S141344
PROQOLID. (2015, April 11). Irritable Bowel Syndrome Quality of Life (IBS-QOL). http://proqolid.org/instruments/irritable_bowel_syndrome_quality_of_life_ibs_qol
Radu, M., Moldovan, R., Pintea, S., Băban, A., & Dumitrascu, D. (2018). Predictors of outcome in cognitive and behavioural interventions for irritable bowel syndrome. A meta-analysis. Journal of gastrointestinal and liver diseases : JGLD, 27(3), 257–263. https://doi.org/10.15403/jgld.2014.1121.273.bab
Radziszewska, M., Smarkusz-Zarzecka, J., & Ostrowska, L. (2023). Nutrition, Physical Activity and Supplementation in Irritable Bowel Syndrome. Nutrients, 15(16), 3662. https://doi.org/10.3390/nu15163662
Rao, S. S. C., Seaton, K., Miller, M., Brown, K., Nygaard, I., Stumbo, P., … Schulze, K. (2007). Randomized Controlled Trial of Biofeedback, Sham Feedback, and Standard Therapy for Dyssynergic Defecation. Clinical Gastroenterology and Hepatology, 5(3), 331–338. doi:10.1016/j.cgh.2006.12.023
Rao, S. S. C., Valestin, J., Brown, K. C., Zimmerman, B., & Schulze, K. (2010). Long-term efficacy of biofeedback therapy for dyssynergic defecation: Randomized controlled trial. American Journal of Gastroenterology, 105(4), 890–896. https://doi.org/10.1038/ajg.2010.53
Raskov, H., Burcharth, J., Pommergaard, H.-C., & Rosenberg, J. (2016). Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes, 7(5), 365–383. https://doi.org/10.1080/19490976.2016.1218585
Rauf, D. (2024, April 19). Healthy lifestyle behaviors can lower the risk of IBS. Everyday Health. https://www.everydayhealth.com/digestive-health/healthy-lifestyle-behaviors-can-lower-the-risk-of-ibs/
Rebel Med NW. (2021). Natural treatment approach to irritable bowel syndrome (IBS). https://www.rebelmednw.com/blog/natural-treatment-approach-to-irritable-bowel-syndrome-ibs
Ryan, M., & Gevirtz, R. (2004). Biofeedback-based psychophysiological treatment in a primary care setting: An initial feasibility study. Applied Psychophysiology and Biofeedback, 29(2), 79–93. https://doi.org/10.1023/B:APBI.0000026635.03016.ef
Saint Luke's Health System. (n.d.). Diet and Lifestyle Tips for Irritable Bowel Syndrome (IBS). Retrieved from https://www.saintlukeskc.org/health-library/diet-and-lifestyle-tips-irritable-bowel-syndrome-ibs
Shaffer, F., & Meehan, Z. M. (2020). A practical guide to resonance frequency assessment for heart rate variability biofeedback. Frontiers in Neuroscience, 14, 570400. https://doi.org/10.3389/fnins.2020.570400
Sharma, A., & Rao, S. (2016). Constipation: Pathophysiology and current therapeutic approaches. In Handbook of experimental pharmacology (Vol. 238, pp. 59–74). Springer. https://doi.org/10.1007/164_2016_111
Sharma, A., Rao, S. S. C., Kearns, K., Orleck, K. D., & Waldman, S. A. (2021). Review article: Diagnosis, management and patient perspectives of the spectrum of constipation disorders. Alimentary Pharmacology & Therapeutics, 53(12), 1250–1267. https://doi.org/10.1111/apt.16369
Simrén, M., Barbara, G., Flint, H. J., Spiegel, B. M. R., Spiller, R. C., Vanner, S., & Zoetendal, E. G. (2012). Intestinal microbiota in functional bowel disorders: A Rome foundation report. Gut, 62(1), 159–176. https://doi.org/10.1136/gutjnl-2012-302167
Sowder, E., Gevirtz, R., Shapiro, W., & Ebert, C. (2010). Restoration of vagal tone: A possible mechanism for functional abdominal pain. Applied Psychophysiology and Biofeedback, 35(3), 199–206. https://doi.org/10.1007/s10484-010-9128-8
Spiller, R., Aziz, Q., Creed, F., Emmanuel, A., Houghton, L., Hungin, A., Jones, R., Kumar, D., Rubin, G., Trudgill, N., Whorwell, P., & Yardley, L. (2007). Guidelines on the irritable bowel syndrome: Mechanisms and practical management. Gut, 56, 1770–1798. https://doi.org/10.1136/gut.2007.119446
Stern, M. J., Guiles, R. A., & Gevirtz, R. (2014). HRV biofeedback for pediatric irritable bowel syndrome and functional abdominal pain: A clinical replication series. Applied Psychophysiology and Biofeedback, 39, 287–291. https://doi.org/10.1007/s10484-014-9261-0
Tillisch, K., Mayer, E. A., Labus, J. S., Stains, J., Chang, L., & Naliboff, B. D. (2005). Sex-specific alterations in autonomic function among patients with irritable bowel syndrome. Gut, 54(10), 1396–1401. https://doi.org/10.1136/gut.2004.060236
Tsigaridas, A., Papanikolaou, I. S., Vaiopoulou, A., Anagnostopoulos, A. K., Viazis, N., Karamanolis, G., Karamanolis, D. G., Tsangaris, G. T., Mantzaris, G. J., & Gazouli, M. (2017). Proteomics and irritable bowel syndrome. Expert Review of Proteomics, 14(5), 461–468. https://doi.org/10.1080/14789450.2017.1317600
Tunali, V., Arslan, N. Ç., Ermiş, B. H., Hakim, G. D., Gündoğdu, A., Hora, M., & Nalbantoğlu, Ö. U. (2024). A multicenter randomized controlled trial of microbiome-based artificial intelligence-assisted personalized diet vs low-fermentable oligosaccharides, disaccharides, monosaccharides, and polyols diet: A novel approach for the management of irritable bowel syndrome. The American Journal of Gastroenterology, 119(9), 1901–1912. https://doi.org/10.14309/ajg.0000000000002862
van Kessel, L., Teunissen, D., & Lagro-Janssen, T. (2021). Sex-gender differences in the effectiveness of treatment of irritable bowel syndrome: A systematic review. International Journal of General Medicine, 14, 867–884. https://doi.org/10.2147/IJGM.S291964
Vezenkov, S. R. (2016). A complex program for prevention of occupational deprivation in persons with irritable bowel syndrome (IBS) [Master's thesis, Ruse University "Angel Kynchev"]. ResearchGate. https://doi.org/10.13140/RG.2.2.10009.75365
Vezenkov, S. R. (2019). Autonomous neurotraining with biofeedback eliminates completely autonomous chess grandmaster symptoms occurring during tournaments in zeitnot (Case Study). ResearchGate. https://doi.org/10.13140/RG.2.2.23177.39527
Vezenkov, S. R. (2019). Successful influence of autonomous dysregulation by biofeedback neurotrainings – Bulgarian practice. ResearchGate. https://doi.org/10.13140/RG.2.2.13277.15848
Vezenkov, S. R., & Mitev, S. O. (2015). A modified brief biofeedback protocol improves the quality of life in a female patient with severe irritable bowel syndrome: A case report. In XI Congress of Bulgarian Society of Physiological Sciences. ResearchGate. https://doi.org/10.13140/RG.2.1.1159.1767
Yang, P.-L., Burr, R. L., de la Iglesia, H. O., Buchanan, D. T., Ward, T. M., & Landis, C. A. (2021). Associations between chronotype, social jetlag, and weekday sleep in women with irritable bowel syndrome. Chronobiology International, 38(5), 742–752. https://doi.org/10.1080/07420528.2021.1885430
Zernicke, K. A., Campbell, T. S., Blustein, P. K., Fung, T. S., Johnson, J. A., Bacon, S. L., & Carlson, L. E. (2013). Mindfulness-based stress reduction for the treatment of irritable bowel syndrome symptoms: A randomized wait-list controlled trial. International Journal of Behavioral Medicine, 20(3), 385–396. https://doi.org/10.1007/s12529-012-9241-6